Enzymatic kinetic resolution of desmethylphosphinothricin indicates that phosphinic group is a bioisostere of carboxyl group
- PMID: 36703359
- PMCID: PMC9814759
- DOI: 10.1038/s42004-020-00368-z
Enzymatic kinetic resolution of desmethylphosphinothricin indicates that phosphinic group is a bioisostere of carboxyl group
Abstract
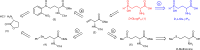
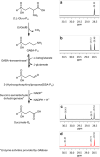
Escherichia coli glutamate decarboxylase (EcGadB), a pyridoxal 5'-phosphate (PLP)-dependent enzyme, is highly specific for L-glutamate and was demonstrated to be effectively immobilised for the production of γ-aminobutyric acid (GABA), its decarboxylation product. Herein we show that EcGadB quantitatively decarboxylates the L-isomer of D,L-2-amino-4-(hydroxyphosphinyl)butyric acid (D,L-Glu-γ-PH), a phosphinic analogue of glutamate containing C-P-H bonds. This yields 3-aminopropylphosphinic acid (GABA-PH), a known GABAB receptor agonist and provides previously unknown D-Glu-γ-PH, allowing us to demonstrate that L-Glu-γ-PH, but not D-Glu-γ-PH, is responsible for D,L-Glu-γ-PH antibacterial activity. Furthermore, using GABase, a preparation of GABA-transaminase and succinic semialdehyde dehydrogenase, we show that GABA-PH is converted to 3-(hydroxyphosphinyl)propionic acid (Succinate-PH). Hence, PLP-dependent and NADP+-dependent enzymes are herein shown to recognise and metabolise phosphinic compounds, leaving unaffected the P-H bond. We therefore suggest that the phosphinic group is a bioisostere of the carboxyl group and the metabolic transformations of phosphinic compounds may offer a ground for prodrug design.
© 2020. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
A Desmethylphosphinothricin Dipeptide Derivative Effectively Inhibits Escherichia coli and Bacillus subtilis Growth.Biomolecules. 2023 Sep 26;13(10):1451. doi: 10.3390/biom13101451. Biomolecules. 2023. PMID: 37892133 Free PMC article.
-
Integrated multi-omics unveil the impact of H-phosphinic analogs of glutamate and α-ketoglutarate on Escherichia coli metabolism.J Biol Chem. 2024 Oct;300(10):107803. doi: 10.1016/j.jbc.2024.107803. Epub 2024 Sep 21. J Biol Chem. 2024. PMID: 39307306 Free PMC article.
-
Enzymatic Synthesis of Biologically Active H-Phosphinic Analogue of α-Ketoglutarate.Biomolecules. 2024 Dec 10;14(12):1574. doi: 10.3390/biom14121574. Biomolecules. 2024. PMID: 39766281 Free PMC article.
-
Inherited disorders of GABA metabolism.J Inherit Metab Dis. 1993;16(4):704-15. doi: 10.1007/BF00711902. J Inherit Metab Dis. 1993. PMID: 8412016 Review.
-
Glutamate Decarboxylase from Lactic Acid Bacteria-A Key Enzyme in GABA Synthesis.Microorganisms. 2020 Dec 3;8(12):1923. doi: 10.3390/microorganisms8121923. Microorganisms. 2020. PMID: 33287375 Free PMC article. Review.
Cited by
-
Synthesis and Biological Activity of Homohypotaurine Obtained by the Enzyme-Based Conversion of Homocysteine Sulfinic Acid Using Recombinant Escherichia Coli Glutamate Decarboxylase.Molecules. 2024 Aug 23;29(17):3985. doi: 10.3390/molecules29173985. Molecules. 2024. PMID: 39274833 Free PMC article.
-
Antibacterial Activity of Peptide Derivatives of Phosphinothricin against Multidrug-Resistant Klebsiella pneumoniae.Molecules. 2023 Jan 27;28(3):1234. doi: 10.3390/molecules28031234. Molecules. 2023. PMID: 36770901 Free PMC article.
-
A Desmethylphosphinothricin Dipeptide Derivative Effectively Inhibits Escherichia coli and Bacillus subtilis Growth.Biomolecules. 2023 Sep 26;13(10):1451. doi: 10.3390/biom13101451. Biomolecules. 2023. PMID: 37892133 Free PMC article.
-
Synthesis of versatile neuromodulatory molecules by a gut microbial glutamate decarboxylase.bioRxiv [Preprint]. 2024 Jun 14:2024.03.02.583032. doi: 10.1101/2024.03.02.583032. bioRxiv. 2024. Update in: iScience. 2025 Mar 25;28(4):112289. doi: 10.1016/j.isci.2025.112289. PMID: 38915512 Free PMC article. Updated. Preprint.
-
Synthesis of versatile neuromodulatory molecules by a gut microbial glutamate decarboxylase.iScience. 2025 Mar 25;28(4):112289. doi: 10.1016/j.isci.2025.112289. eCollection 2025 Apr 18. iScience. 2025. PMID: 40264799 Free PMC article.
References
-
- Fonda M. Glutamate decarboxylase. Substrate specificity and inhibition by carboxylic acids. Biochemistry. 1972;11:1304–1309. - PubMed
-
- Fonda ML. L-Glutamate decarboxylase from bacteria. Methods Enzymol. 1985;113:11–16. - PubMed
-
- Sukhareva, B. S. Amino acid decarboxylases. PyridoxaL Phosphate: Chemical, Biochemical and Medical Aspects Vol. 1B, 325–353 (Wiley, NY, 1986).
-
- Lammens TM, De Biase D, Franssen MCR, Scott EL, Sanders JPM. The application of glutamic acid alpha-decarboxylase for the valorization of glutamic acid. Green Chem. 2009;11:1562–1567.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

