Single step syntheses of (1S)-aryl-tetrahydroisoquinolines by norcoclaurine synthases
- PMID: 36703392
- PMCID: PMC9814250
- DOI: 10.1038/s42004-020-00416-8
Single step syntheses of (1S)-aryl-tetrahydroisoquinolines by norcoclaurine synthases
Abstract
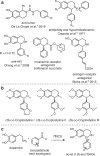
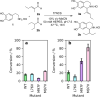
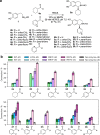
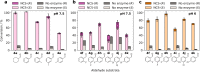
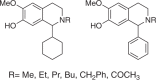
The 1-aryl-tetrahydroisoquinoline (1-aryl-THIQ) moiety is found in many biologically active molecules. Single enantiomer chemical syntheses are challenging and although some biocatalytic routes have been reported, the substrate scope is limited to certain structural motifs. The enzyme norcoclaurine synthase (NCS), involved in plant alkaloid biosynthesis, has been shown to perform stereoselective Pictet-Spengler reactions between dopamine and several carbonyl substrates. Here, benzaldehydes are explored as substrates and found to be accepted by both wild-type and mutant constructs of NCS. In particular, the variant M97V gives a range of (1 S)-aryl-THIQs in high yields (48-99%) and e.e.s (79-95%). A co-crystallised structure of the M97V variant with an active site reaction intermediate analogue is also obtained with the ligand in a pre-cyclisation conformation, consistent with (1 S)-THIQs formation. Selected THIQs are then used with catechol O-methyltransferases with exceptional regioselectivity. This work demonstrates valuable biocatalytic approaches to a range of (1 S)-THIQs.
© 2020. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Engineering a norcoclaurine synthase for one-step synthesis of (S)-1-aryl-tetrahydroisoquinolines.Bioresour Bioprocess. 2023 Mar 1;10(1):15. doi: 10.1186/s40643-023-00637-4. Bioresour Bioprocess. 2023. PMID: 38647611 Free PMC article.
-
Asymmetric synthesis of tetrahydroisoquinolines by enzymatic Pictet-Spengler reaction.Biosci Biotechnol Biochem. 2014;78(4):701-7. doi: 10.1080/09168451.2014.890039. Epub 2014 Apr 29. Biosci Biotechnol Biochem. 2014. PMID: 25036970
-
Enzyme catalysed Pictet-Spengler formation of chiral 1,1'-disubstituted- and spiro-tetrahydroisoquinolines.Nat Commun. 2017 Apr 3;8:14883. doi: 10.1038/ncomms14883. Nat Commun. 2017. PMID: 28368003 Free PMC article.
-
Occurrence of Enantioselectivity in Nature: The Case of (S)-Norcoclaurine.Chirality. 2016 Mar;28(3):169-80. doi: 10.1002/chir.22566. Epub 2016 Jan 5. Chirality. 2016. PMID: 26729048 Review.
-
Norcoclaurine synthase: mechanism of an enantioselective pictet-spengler catalyzing enzyme.Molecules. 2010 Mar 24;15(4):2070-8. doi: 10.3390/molecules15042070. Molecules. 2010. PMID: 20428026 Free PMC article. Review.
Cited by
-
Understanding the Enzyme (S)-Norcoclaurine Synthase Promiscuity to Aldehydes and Ketones.J Chem Inf Model. 2024 Jun 10;64(11):4462-4474. doi: 10.1021/acs.jcim.3c01773. Epub 2024 May 22. J Chem Inf Model. 2024. PMID: 38776464 Free PMC article.
-
Methyltransferases: Functions and Applications.Chembiochem. 2022 Sep 16;23(18):e202200212. doi: 10.1002/cbic.202200212. Epub 2022 Jul 5. Chembiochem. 2022. PMID: 35691829 Free PMC article. Review.
-
Highly Acidic Electron-Rich Brønsted Acids Accelerate Asymmetric Pictet-Spengler Reactions by Virtue of Stabilizing Cation-π Interactions.J Am Chem Soc. 2024 Oct 3;146(41):28339-49. doi: 10.1021/jacs.4c09421. Online ahead of print. J Am Chem Soc. 2024. PMID: 39361889 Free PMC article.
-
New Trends and Future Opportunities in the Enzymatic Formation of C-C, C-N, and C-O bonds.Chembiochem. 2022 Mar 18;23(6):e202100464. doi: 10.1002/cbic.202100464. Epub 2021 Nov 24. Chembiochem. 2022. PMID: 34726813 Free PMC article. Review.
-
Engineering a norcoclaurine synthase for one-step synthesis of (S)-1-aryl-tetrahydroisoquinolines.Bioresour Bioprocess. 2023 Mar 1;10(1):15. doi: 10.1186/s40643-023-00637-4. Bioresour Bioprocess. 2023. PMID: 38647611 Free PMC article.
References
-
- Burks, H. E. et al. 1,2,3,4-Tetrahydroisoquinoline compounds and compositions as selective estrogen receptor antagonists and degraders. Patent WO2015092634A1 (2015).
-
- Coppola, J. A., Paul, R. & Cohen, E. 1-(4’-Substituted-phenyl)-2-(phenyl lower alkyl)-1,2,3,4-tetrahydroisoquinolines. US patent 3,597,431 (1971).
Grants and funding
LinkOut - more resources
Full Text Sources

