Structural characterisation of molecular conformation and the incorporation of adatoms in an on-surface Ullmann-type reaction
- PMID: 36703404
- PMCID: PMC9814584
- DOI: 10.1038/s42004-020-00402-0
Structural characterisation of molecular conformation and the incorporation of adatoms in an on-surface Ullmann-type reaction
Abstract
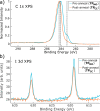
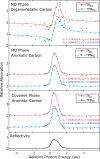
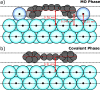
The on-surface synthesis of covalently bonded materials differs from solution-phase synthesis in several respects. The transition from a three-dimensional reaction volume to quasi-two-dimensional confinement, as is the case for on-surface synthesis, has the potential to facilitate alternative reaction pathways to those available in solution. Ullmann-type reactions, where the surface plays a role in the coupling of aryl-halide functionalised species, has been shown to facilitate extended one- and two-dimensional structures. Here we employ a combination of scanning tunnelling microscopy (STM), X-ray photoelectron spectroscopy (XPS) and X-ray standing wave (XSW) analysis to perform a chemical and structural characterisation of the Ullmann-type coupling of two iodine functionalised species on a Ag(111) surface held under ultra-high vacuum (UHV) conditions. Our results allow characterisation of molecular conformations and adsorption geometries within an on-surface reaction and provide insight into the incorporation of metal adatoms within the intermediate structures of the reaction.
© 2020. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Shen Q, Gao H-Y, Fuchs H. Frontiers of on-surface synthesis: from principles to applications. Nano Today. 2017;13:77–96. doi: 10.1016/j.nantod.2017.02.007. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

