N-Heterocyclic carbene-catalyzed enantioselective hetero-[10 + 2] annulation
- PMID: 36703423
- PMCID: PMC9814252
- DOI: 10.1038/s42004-020-00425-7
N-Heterocyclic carbene-catalyzed enantioselective hetero-[10 + 2] annulation
Abstract
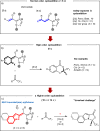
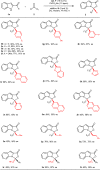
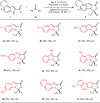
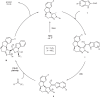
Higher-order cycloadditions are a powerful strategy for the construction of polycycles in one step. However, an efficient and concise version for the induction of asymmetry is lacking. N-heterocyclic carbenes are widely used organocatalysts for asymmetric synthesis and could be an ideal choice for enantioselective higher-order cycloadditions. Here, we report an enantioselective [10 + 2] annulation between catalytically formed aza-benzofulvene intermediates and trifluoromethyl ketone derivatives. This protocol exhibits a wide scope, high yields, and good ee values, reflecting a robust and efficient higher-order cycloaddition. Density functional theory calculations provide an accurate prediction of the reaction enantioselectivity, and in-depth insight to the origins of stereocontrol.
© 2020. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Carbene-Catalyzed Enantioselective Decarboxylative Annulations to Access Dihydrobenzoxazinones and Quinolones.Angew Chem Int Ed Engl. 2019 Apr 23;58(18):5941-5945. doi: 10.1002/anie.201900600. Epub 2019 Mar 27. Angew Chem Int Ed Engl. 2019. PMID: 30843323 Free PMC article.
-
Asymmetric N-Heterocyclic Carbene Catalyzed Annulation of 2-Alkenylbenzothiazoles with α-Chloro Aldehydes.Synthesis (Stuttg). 2014 Nov 6;47(3):421-428. doi: 10.1055/s-0034-1379369. Synthesis (Stuttg). 2014. PMID: 26752795 Free PMC article.
-
N-heterocyclic carbene catalyzed formal [3+2] annulation reaction of enals: an efficient enantioselective access to spiro-heterocycles.Angew Chem Int Ed Engl. 2014 Sep 15;53(38):10232-6. doi: 10.1002/anie.201405381. Epub 2014 Aug 11. Angew Chem Int Ed Engl. 2014. PMID: 25130870
-
N-Heterocyclic Carbene Catalysis via Azolium Dienolates: An Efficient Strategy for Remote Enantioselective Functionalizations.Angew Chem Int Ed Engl. 2018 Apr 3;57(15):3862-3873. doi: 10.1002/anie.201709684. Epub 2018 Mar 9. Angew Chem Int Ed Engl. 2018. PMID: 29136320 Review.
-
Bifunctional N-Heterocyclic Carbenes Derived from l-Pyroglutamic Acid and Their Applications in Enantioselective Organocatalysis.Acc Chem Res. 2020 Mar 17;53(3):690-702. doi: 10.1021/acs.accounts.9b00635. Epub 2020 Mar 6. Acc Chem Res. 2020. PMID: 32142245 Review.
Cited by
-
Biomimetic enantioselective synthesis of β,β-difluoro-α-amino acid derivatives.Commun Chem. 2021 Oct 22;4(1):148. doi: 10.1038/s42004-021-00586-z. Commun Chem. 2021. PMID: 36697625 Free PMC article.
-
Dynamic kinetic resolution of γ,γ-disubstituted indole 2-carboxaldehydes via NHC-Lewis acid cooperative catalysis for the synthesis of tetracyclic ε-lactones.Chem Sci. 2022 Aug 29;13(39):11513-11518. doi: 10.1039/d2sc03745a. eCollection 2022 Oct 12. Chem Sci. 2022. PMID: 36320396 Free PMC article.
-
Asymmetric higher-order [10 + n] cycloadditions of palladium-containing 10π-cycloaddends.Chem Sci. 2022 Jul 15;13(32):9265-9270. doi: 10.1039/d2sc02985e. eCollection 2022 Aug 17. Chem Sci. 2022. PMID: 36092999 Free PMC article.
References
LinkOut - more resources
Full Text Sources

