N-Heterocyclic carbene-catalyzed enantioselective hetero-[10 + 2] annulation
- PMID: 36703423
- PMCID: PMC9814252
- DOI: 10.1038/s42004-020-00425-7
N-Heterocyclic carbene-catalyzed enantioselective hetero-[10 + 2] annulation
Abstract
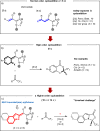
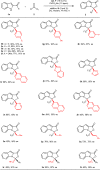
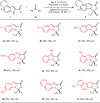
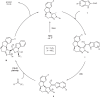
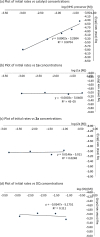
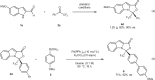
Higher-order cycloadditions are a powerful strategy for the construction of polycycles in one step. However, an efficient and concise version for the induction of asymmetry is lacking. N-heterocyclic carbenes are widely used organocatalysts for asymmetric synthesis and could be an ideal choice for enantioselective higher-order cycloadditions. Here, we report an enantioselective [10 + 2] annulation between catalytically formed aza-benzofulvene intermediates and trifluoromethyl ketone derivatives. This protocol exhibits a wide scope, high yields, and good ee values, reflecting a robust and efficient higher-order cycloaddition. Density functional theory calculations provide an accurate prediction of the reaction enantioselectivity, and in-depth insight to the origins of stereocontrol.
© 2020. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources

