Sigmoidally hydrochromic molecular porous crystal with rotatable dendrons
- PMID: 36703455
- PMCID: PMC9814496
- DOI: 10.1038/s42004-020-00364-3
Sigmoidally hydrochromic molecular porous crystal with rotatable dendrons
Abstract
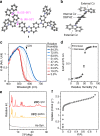
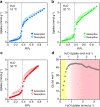
Vapochromic behaviour of porous crystals is beneficial for facile and rapid detection of gaseous molecules without electricity. Toward this end, tailored molecular designs have been established for metal-organic, covalent-bonded and hydrogen-bonded frameworks. Here, we explore the hydrochromic chemistry of a van der Waals (VDW) porous crystal. The VDW porous crystal VPC-1 is formed from a novel aromatic dendrimer having a dibenzophenazine core and multibranched carbazole dendrons. Although the constituent molecules are connected via VDW forces, VPC-1 maintains its structural integrity even after desolvation. VPC-1 exhibits reversible colour changes upon uptake/release of water molecules due to the charge transfer character of the constituent dendrimer. Detailed structural analyses reveal that the outermost carbazole units alone are mobile in the crystal and twist simultaneously in response to water vapour. Thermodynamic analysis suggests that the sigmoidal water sorption is induced by the affinity alternation of the pore surface from hydrophobic to hydrophilic.
© 2020. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Daws CA, Exstrom CL, Sowa JR, Mann KR. “Vapochromic” compounds as environmental sensors. 2. Synthesis and near-infrared and infrared spectroscopy studies of [Pt(arylisocyanide)4][Pt(CN)4] upon exposure to volatile organic compound vapors. Chem. Mater. 1997;9:363–368. doi: 10.1021/cm960397z. - DOI
-
- Cariati E, Bu X, Ford PC. Solvent- and vapor-induced isomerization between the luminescent solids [CuI(4-pic)]4 and [CuI(4-pic)]∞ (pic = methylpyridine). The structural basis for the observed luminescence vapochromism. Chem. Mater. 2000;12:3385–3391. doi: 10.1021/cm0010708. - DOI
Grants and funding
- JP19K15334/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP17H05155/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP19H05716/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP19H02746/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP17H05146/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP15H05757/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP19H05717/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP16H06521/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP20H00369/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP17H05142/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP16H02081/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP15KK0182/MEXT | Japan Society for the Promotion of Science (JSPS)
- UMA18-FEDERJA-080/Junta de Andalucía
LinkOut - more resources
Full Text Sources

