Alpha-adrenergic receptor activation after fetal hypoxia-ischaemia suppresses transient epileptiform activity and limits loss of oligodendrocytes and hippocampal neurons
- PMID: 36703575
- PMCID: PMC10196751
- DOI: 10.1177/0271678X231153723
Alpha-adrenergic receptor activation after fetal hypoxia-ischaemia suppresses transient epileptiform activity and limits loss of oligodendrocytes and hippocampal neurons
Abstract
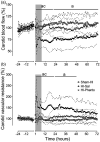
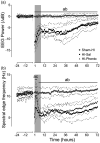
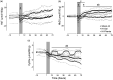
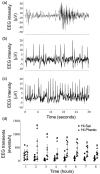
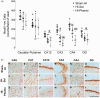
Exposure to hypoxic-ischaemia (HI) is consistently followed by a delayed fall in cerebral perfusion. In preterm fetal sheep this is associated with impaired cerebral oxygenation, consistent with mismatch between perfusion and metabolism. In the present study we tested the hypothesis that alpha-adrenergic inhibition after HI would improve cerebral perfusion, and so attenuate mismatch and reduce neural injury. Chronically instrumented preterm (0.7 gestation) fetal sheep received sham-HI (n = 10) or HI induced by complete umbilical cord occlusion for 25 minutes. From 15 minutes to 8 hours after HI, fetuses received either an intravenous infusion of a non-selective alpha-adrenergic antagonist, phentolamine (10 mg bolus, 10 mg/h infusion, n = 10), or saline (n = 10). Fetal brains were processed for histology 72 hours post-HI. Phentolamine infusion was associated with increased epileptiform transient activity and a greater fall in cerebral oxygenation in the early post-HI recovery phase. Histologically, phentolamine was associated with greater loss of oligodendrocytes and hippocampal neurons. In summary, contrary to our hypothesis, alpha-adrenergic inhibition increased epileptiform transient activity with an exaggerated fall in cerebral oxygenation, and increased neural injury, suggesting that alpha-adrenergic receptor activation after HI is an important endogenous neuroprotective mechanism.
Keywords: Hypoxia-ischaemia; cerebral hypoperfusion; neural injury; neuro-inhibition; sympathetic nervous system.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Adverse neural effects of delayed, intermittent treatment with rEPO after asphyxia in preterm fetal sheep.J Physiol. 2021 Jul;599(14):3593-3609. doi: 10.1113/JP281269. Epub 2021 Jun 18. J Physiol. 2021. PMID: 34032286
-
Endogenous alpha2-adrenergic receptor-mediated neuroprotection after severe hypoxia in preterm fetal sheep.Neuroscience. 2006 Oct 27;142(3):615-28. doi: 10.1016/j.neuroscience.2006.06.066. Epub 2006 Sep 6. Neuroscience. 2006. PMID: 16952424
-
Impact of hypoxia-ischemia and dopamine treatment on dopamine receptor binding density in the preterm fetal sheep brain.J Appl Physiol (1985). 2020 Dec 1;129(6):1431-1438. doi: 10.1152/japplphysiol.00677.2020. Epub 2020 Oct 15. J Appl Physiol (1985). 2020. PMID: 33054660
-
Preterm umbilical cord blood derived mesenchymal stem/stromal cells protect preterm white matter brain development against hypoxia-ischemia.Exp Neurol. 2018 Oct;308:120-131. doi: 10.1016/j.expneurol.2018.07.006. Epub 2018 Jul 20. Exp Neurol. 2018. PMID: 30012511
-
Cerebral Oxygenation and Metabolism After Hypoxia-Ischemia.Front Pediatr. 2022 Jul 12;10:925951. doi: 10.3389/fped.2022.925951. eCollection 2022. Front Pediatr. 2022. PMID: 35903161 Free PMC article. Review.
Cited by
-
Impact of hypoxia on the hippocampus: A review.Medicine (Baltimore). 2025 Mar 21;104(12):e41479. doi: 10.1097/MD.0000000000041479. Medicine (Baltimore). 2025. PMID: 40128035 Free PMC article. Review.
-
Circadian patterns of heart rate variability in fetal sheep after hypoxia-ischaemia: A biomarker of evolving brain injury.J Physiol. 2024 Dec;602(23):6553-6569. doi: 10.1113/JP284560. Epub 2023 Jul 11. J Physiol. 2024. PMID: 37432936 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

