Transferrin Receptor-Mediated Iron Uptake Promotes Colon Tumorigenesis
- PMID: 36703617
- PMCID: PMC10074045
- DOI: 10.1002/advs.202207693
Transferrin Receptor-Mediated Iron Uptake Promotes Colon Tumorigenesis
Abstract
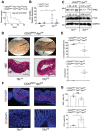
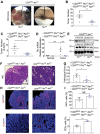
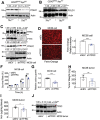
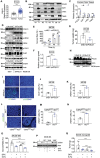
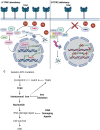
Transferrin receptor (TFRC) is the major mediator for iron entry into a cell. Under excessive iron conditions, TFRC is expected to be reduced to lower iron uptake and toxicity. However, the mechanism whereby TFRC expression is maintained at high levels in iron-enriched cancer cells and the contribution of TFRC to cancer development are enigmatic. Here the work shows TFRC is induced by adenomatous polyposis coli (APC) gene loss-driven β-catenin activation in colorectal cancer, whereas TFRC-mediated intratumoral iron accumulation potentiates β-catenin signaling by directly enhancing the activity of tankyrase. Disruption of TFRC leads to a reduction of colonic iron levels and iron-dependent tankyrase activity, which caused stabilization of axis inhibition protein 2 (AXIN2) and subsequent repression of the β-catenin/c-Myc/E2F Transcription Factor 1/DNA polymerase delta1 (POLD1) axis. POLD1 knockdown, iron chelation, and TFRC disruption increase DNA replication stress, DNA damage response, apoptosis, and reduce colon tumor growth. Importantly, a combination of iron chelators and DNA damaging agents increases DNA damage response and reduces colon tumor cell growth. TFRC-mediated iron import is at the center of a novel feed-forward loop that facilitates colonic epithelial cell survival. This discovery may provide novel strategies for colorectal cancer therapy.
Keywords: DNA damage response; TFRC; colon; iron; β-catenin.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
C.A.L. has received consulting fees from Astellas Pharmaceuticals and Odyssey Therapeutics and is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in cancer, and targeting the GOT1‐pathway as a therapeutic approach.
Figures


References
-
- Siegel R. L., Miller K. D., Fuchs H. E., Jemal A., CA Cancer J. Clin. 2021, 71, 7. - PubMed
-
- Soerjomataram I., Bray F., Nat. Rev. Clin. Oncol. 2021, 18, 663. - PubMed
-
- Bastide N. M., Chenni F., Audebert M., Santarelli R. L., Taché S., Naud N., Baradat M., Jouanin I., Surya R., Hobbs D. A., Kuhnle G. G., Letron I. R., Gueraud F., Corpet D. E., Pierre F. H. F., Cancer Res. 2015, 75, 870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA118100/CA/NCI NIH HHS/United States
- P30CA118100/BC/NCI NIH HHS/United States
- P20 GM130422/NH/NIH HHS/United States
- R01 ES028802/ES/NIEHS NIH HHS/United States
- U01 DK103141/DK/NIDDK NIH HHS/United States
- R01 CA244931/CA/NCI NIH HHS/United States
- R01ES028802/ES/NIEHS NIH HHS/United States
- P30 ES017885/ES/NIEHS NIH HHS/United States
- K12-GM088021/GM/NIGMS NIH HHS/United States
- K12 GM088021/GM/NIGMS NIH HHS/United States
- R01CA244931/BC/NCI NIH HHS/United States
- P20 GM130422/GM/NIGMS NIH HHS/United States
- P30ES017885/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
