Maternal sevoflurane exposure induces neurotoxicity in offspring rats via the CB1R/CDK5/p-tau pathway
- PMID: 36703741
- PMCID: PMC9871255
- DOI: 10.3389/fphar.2022.1066713
Maternal sevoflurane exposure induces neurotoxicity in offspring rats via the CB1R/CDK5/p-tau pathway
Abstract
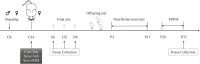
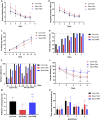
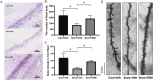
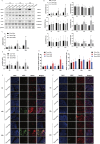
Sevoflurane is widely used for maternal anesthesia during pregnancy. Sevoflurane exposure of rats at mid-gestation can cause abnormal development of the central nervous system in their offspring. Sevoflurane is known to increase the expression of cannabinoid 1 receptor (CB1R) in the hippocampus. However, the effect of cannabinoid 1 receptor on fetal and offspring rats after maternal anesthesia is still unclear. At gestational day 14, pregnant rats were subjected to 2-h exposure to 3.5% sevoflurane or air. Rats underwent intraperitoneal injection with saline or rimonabant (1 mg/kg) 30 min prior to sevoflurane or air exposure. cannabinoid 1 receptor, cyclin-dependent kinase 5 (CDK5), p35, p25, tau, and p-tau expression in fetal brains was measured at 6, 12, and 24 h post-sevoflurane/air exposure. Neurobehavioral and Morris water maze tests were performed postnatal days 3-33. The expression of cannabinoid 1 receptor/cyclin-dependent kinase 5/p-tau and histopathological staining of brain tissues in offspring rats was observed. We found that a single exposure to sevoflurane upregulated the activity of cyclin-dependent kinase 5 and the level of p-tau via cannabinoid 1 receptor. This was accompanied by the diminished number of neurons and dendritic spines in hippocampal CA1 regions. Finally, these effects induced lower scores and platform crossing times in behavioral tests. The present study suggests that a single exposure to 3.5% sevoflurane of rats at mid-gestation impairs neurobehavioral abilities and cognitive memory in offspring. cannabinoid 1 receptor is a possible target for the amelioration of postnatal neurobehavioral ability and cognitive memory impairments induced by maternal anesthesia.
Keywords: CB1R; hippocampus; learning and memory; rimonabant; sevoflurane; tau.
Copyright © 2023 Wan, Wu, Li and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Mid-gestational sevoflurane exposure inhibits fetal neural stem cell proliferation and impairs postnatal learning and memory function in a dose-dependent manner.Dev Biol. 2018 Mar 15;435(2):185-197. doi: 10.1016/j.ydbio.2018.01.022. Epub 2018 Feb 2. Dev Biol. 2018. PMID: 29410165
-
Suberoylanilide hydroxamic acid (SAHA) alleviates the learning and memory impairment in rat offspring caused by maternal sevoflurane exposure during late gestation.J Toxicol Sci. 2019;44(3):177-189. doi: 10.2131/jts.44.177. J Toxicol Sci. 2019. PMID: 30842370
-
Regulation of CRMP2 by Cdk5 and GSK-3β participates in sevoflurane-induced dendritic development abnormalities and cognitive dysfunction in developing rats.Toxicol Lett. 2021 May 1;341:68-79. doi: 10.1016/j.toxlet.2021.01.023. Epub 2021 Feb 3. Toxicol Lett. 2021. PMID: 33548343
-
Effects of Sevoflurane Exposure During Mid-Pregnancy on Learning and Memory in Offspring Rats: Beneficial Effects of Maternal Exercise.Front Cell Neurosci. 2018 May 3;12:122. doi: 10.3389/fncel.2018.00122. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29773978 Free PMC article.
-
Prenatal sevoflurane exposure: Effects of iron metabolic dysfunction on offspring cognition and potential mechanism.Int J Dev Neurosci. 2021 Feb;81(1):1-9. doi: 10.1002/jdn.10080. Epub 2020 Dec 14. Int J Dev Neurosci. 2021. PMID: 33259670 Review.
Cited by
-
Dexmedetomidine Improves Long-term Neurological Outcomes by Promoting Oligodendrocyte Genesis and Myelination in Neonatal Rats Following Hypoxic-ischemic Brain Injury.Mol Neurobiol. 2025 Apr;62(4):4866-4880. doi: 10.1007/s12035-024-04564-z. Epub 2024 Nov 4. Mol Neurobiol. 2025. PMID: 39496877
References
-
- Busquets-Garcia A., Gomis-González M., Srivastava R. K., Cutando L., Ortega-Alvaro A., Ruehle S., et al. (2016). Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Pro Natl. Acad. Sci. U. S. A. 113 (35), 9904–9909. 10.1073/pnas.1525066113 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

