A novel optical imaging probe for targeted visualization of NLRP3 inflammasomes in a mouse model of age-related macular degeneration
- PMID: 36703888
- PMCID: PMC9871584
- DOI: 10.3389/fmed.2022.1047791
A novel optical imaging probe for targeted visualization of NLRP3 inflammasomes in a mouse model of age-related macular degeneration
Abstract
Purpose: Wet form of age-related macular degeneration (wet AMD) is a progressive vascular disease that mainly affects older adults and causes severe and irreversible vision loss. A key complication of wet AMD is choroidal neovascularization (CNV), which may be driven in part by NLRP3 inflammasomes that are associated with macrophages migration to CNV lesions. Since activated NLRP3 is correlated with CNV, visualizing NLRP3 inflammasomes and their associated macrophages is of great interest to monitor wet AMD progression and develop effective therapies against it. However, to the best of our knowledge, current ophthalmic imaging systems do not permit such targeted imaging. Therefore, in this study, we developed InflammaProbe-1, an optical imaging probe for targeted visualization of NLRP3 inflammasomes in CNV lesions.
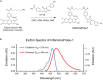
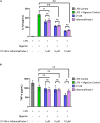
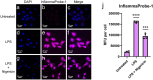
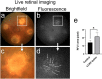
Methods: InflammaProbe-1 was synthesized by conjugating a clinically relevant fluorophore, Oregon Green® 488, to the selective NLRP3 inhibitor, CY-09. The ability of InflammaProbe-1 to target NLRP3 was assessed with an enzyme-linked immunosorbent assay by comparing its ability to inhibit NLRP3-mediated secretion of IL-1β to that of CY-09 in LPS-primed and nigericin-stimulated BMDMs. In vitro confocal imaging of NLRP3 was performed on InflammaProbe-1-stained BMDMs that had been induced to express NLRP3 with LPS. In vivo imaging of NLRP3 was conducted on mouse laser induced choroidal neovascularization (LCNV), a model of AMD, 6 h after an intraperitoneal injection of InflammaProbe-1 at 10 mg/kg on day 4 post-LCNV.
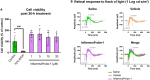
Results: InflammaProbe-1 was just as effective as CY-09 at inhibiting IL-1β secretion (p < 0.01 at 10 μM for both the InflammaProbe-1 and CY-09 groups relative to the control). InflammaProbe-1-stained BMDMs that had been induced to express NLRP3 showed significantly brighter fluorescence than untreated cells (p < 0.0001 for LPS treatment group and p < 0.001 for LPS and nigericin treatment group). Furthermore, in vivo molecular imaging of NLRP3 was achieved in mouse LCNV.
Conclusion: We propose that InflammaProbe-1 may be a useful molecular imaging probe to monitor the onset, progression, and therapeutic response of AMD and other NLRP3-mediated diseases.
Keywords: CY-09; NLRP3 inflammasome; age-related macular degeneration; choroidal neovascularization; macrophages; optical imaging.
Copyright © 2023 Paguaga, Penn and Uddin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Role of NLRP3 Inflammasomes in Monocyte and Microglial Recruitments in Choroidal Neovascularization.Immunohorizons. 2024 May 1;8(5):363-370. doi: 10.4049/immunohorizons.2400025. Immunohorizons. 2024. PMID: 38775688 Free PMC article.
-
Novel Optical Imaging Probe for the Targeted Visualization of NLRP3 Inflammasomes in Living Retina.J Med Chem. 2025 Aug 14;68(15):16034-16047. doi: 10.1021/acs.jmedchem.5c00999. Epub 2025 Aug 4. J Med Chem. 2025. PMID: 40758602 Free PMC article.
-
Role of NLRP3 inflammasomes in monocyte and microglial recruitments in choroidal neovascularization.Res Sq [Preprint]. 2023 Sep 9:rs.3.rs-3318233. doi: 10.21203/rs.3.rs-3318233/v1. Res Sq. 2023. Update in: Immunohorizons. 2024 May 1;8(5):363-370. doi: 10.4049/immunohorizons.2400025. PMID: 37720026 Free PMC article. Updated. Preprint.
-
Role of inflammasome activation in neovascular age-related macular degeneration.FEBS J. 2023 Jan;290(1):28-36. doi: 10.1111/febs.16278. Epub 2021 Dec 4. FEBS J. 2023. PMID: 34767301 Free PMC article. Review.
-
NLRP3 Inflammasome and Pathobiology in AMD.J Clin Med. 2015 Jan 14;4(1):172-92. doi: 10.3390/jcm4010172. J Clin Med. 2015. PMID: 26237026 Free PMC article. Review.
Cited by
-
Role of NLRP3 Inflammasomes in Monocyte and Microglial Recruitments in Choroidal Neovascularization.Immunohorizons. 2024 May 1;8(5):363-370. doi: 10.4049/immunohorizons.2400025. Immunohorizons. 2024. PMID: 38775688 Free PMC article.
-
Novel Optical Imaging Probe for the Targeted Visualization of NLRP3 Inflammasomes in Living Retina.J Med Chem. 2025 Aug 14;68(15):16034-16047. doi: 10.1021/acs.jmedchem.5c00999. Epub 2025 Aug 4. J Med Chem. 2025. PMID: 40758602 Free PMC article.
References
-
- Berglin L. Choroidal neovascularization in age-related macular degeneration?-from mice to man. In: Penn JS. editor. Retinal and Choroidal Angiogenesis. Berlin: Springer; (2008). p. 527–43. 10.1007/978-1-4020-6780-8_24 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

