Inflammatory status in pediatric sickle cell disease: Unravelling the role of immune cell subsets
- PMID: 36703915
- PMCID: PMC9871358
- DOI: 10.3389/fmolb.2022.1075686
Inflammatory status in pediatric sickle cell disease: Unravelling the role of immune cell subsets
Abstract
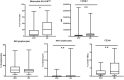
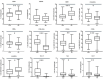
Introduction: The mutation of the beta-globin gene that causes sickle cell disease (SCD) results in pleiotropic effects, such as hemolysis and vaso-occlusive crisis that can induce inflammatory mechanisms with deleterious consequences on the organism. Moreover, SCD patients display an increased susceptibility to infections. Few studies are currently available that evaluate a wide immunological profile in a pediatric population. This study proposes an evaluation of the immune profile in subjects with SCD in a pediatric population through a detailed analysis by flow cytometry. Methods and Materials: Peripheral blood samples from 53 pediatric patients with SCD (mean age 9.8 years, interquartile range 9 years) were obtained and then analyzed by flow cytometry, in order to evaluate changes in the immune populations compared to 40 healthy donors (mean age 7.3 years, interquartile range 9.5 years). Results: Our data showed an increase in neutrophils (with a reduction in the CD62L + subpopulation) and monocytes (with a decrease in HLA-DRlow monocytes) with normal values of lymphocytes in SCD patients. In the lymphocyte subpopulations analysis we observed lower values of CD4+ T cells (with higher number of memory and central memory T lymphocytes) with increased frequency of CD8+ T cells (with a predominant naive pattern). Moreover, we observed higher values of CD39+ Tregs and lower HLA-DR+ and CD39- T cells with an increased Th17, Th1-17 and Th2 response. Conclusion: We observed immunological alterations typical of an inflammatory status (increase in activated neutrophils and monocytes) associated with a peculiar Treg pattern (probably linked to a body attempt to minimize inflammation intrinsic to SCD). Furthermore, we highlighted a T helper pathway associated with inflammation in line with other studies. Our data showed that immunological markers may have an important role in the understanding the pathophysiology of SCD and in optimizing targeted therapeutic strategies for each patient.
Keywords: anemia; flow cytofluorimetry; hemoglobinopathies; immune system; immunophenotype; sickle cell disease.
Copyright © 2023 Marchesani, Bertaina, Marini, Cossutta, Di Mauro, Rotulo, Palma, Sabatini, Petrone, Frati, Monteleone, Palumbo and Ceglie.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Characterizing the Immature Immunophenotype of Sickle Cell Disease Monocytes.Cureus. 2024 May 20;16(5):e60703. doi: 10.7759/cureus.60703. eCollection 2024 May. Cureus. 2024. PMID: 38899253 Free PMC article.
-
Pattern of serum cytokine expression and T-cell subsets in sickle cell disease patients in vaso-occlusive crisis.Clin Vaccine Immunol. 2010 Apr;17(4):602-8. doi: 10.1128/CVI.00145-09. Epub 2010 Feb 3. Clin Vaccine Immunol. 2010. PMID: 20130127 Free PMC article.
-
Altered T-cell profile in sickle cell disease.Biomark Med. 2023 Mar;17(5):241-252. doi: 10.2217/bmm-2023-0086. Epub 2023 May 19. Biomark Med. 2023. PMID: 37204241
-
The Red Blood Cell-Inflammation Vicious Circle in Sickle Cell Disease.Front Immunol. 2020 Mar 13;11:454. doi: 10.3389/fimmu.2020.00454. eCollection 2020. Front Immunol. 2020. PMID: 32231672 Free PMC article. Review.
-
Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis.Front Immunol. 2014 Apr 22;5:180. doi: 10.3389/fimmu.2014.00180. eCollection 2014. Front Immunol. 2014. PMID: 24795723 Free PMC article. Review.
Cited by
-
Impaired phagocytosis and oxidative respiratory burst activity in sickle cell anemia leukocytes.J Taibah Univ Med Sci. 2024 Aug 10;19(4):867-876. doi: 10.1016/j.jtumed.2024.07.010. eCollection 2024 Aug. J Taibah Univ Med Sci. 2024. PMID: 39247449 Free PMC article.
-
Characterizing the Immature Immunophenotype of Sickle Cell Disease Monocytes.Cureus. 2024 May 20;16(5):e60703. doi: 10.7759/cureus.60703. eCollection 2024 May. Cureus. 2024. PMID: 38899253 Free PMC article.
-
HLA haplotype frequencies and diversity in patients with hemoglobinopathies.EJHaem. 2023 Aug 4;4(4):963-969. doi: 10.1002/jha2.763. eCollection 2023 Nov. EJHaem. 2023. PMID: 38024588 Free PMC article.
-
Safety and efficacy studies of CRISPR-Cas9 treatment of sickle cell disease highlights disease-specific responses.Mol Ther. 2024 Dec 4;32(12):4337-4352. doi: 10.1016/j.ymthe.2024.07.015. Epub 2024 Jul 22. Mol Ther. 2024. PMID: 39044427
-
Alterations of T Cell Subsets Associated with Sickle Cell Trait.Blood Genom Discov. 2025;9(1):10001. doi: 10.70322/bgd.2025.10001. Epub 2024 Oct 8. Blood Genom Discov. 2025. PMID: 39720621 Free PMC article.
References
-
- Abraham A. A., Lang H., Meier E. R., Nickel R. S., Dean M., Lawal N., et al. (2019). Characterization of natural killer cells expressing markers associated with maturity and cytotoxicity in children and young adults with sickle cell disease. Pediatr. Blood Cancer 66, e27601. 10.1002/pbc.27601 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

