Natural alleles of the clock gene timeless differentially affect life-history traits in Drosophila
- PMID: 36703932
- PMCID: PMC9871817
- DOI: 10.3389/fphys.2022.1092951
Natural alleles of the clock gene timeless differentially affect life-history traits in Drosophila
Abstract
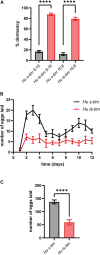
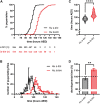
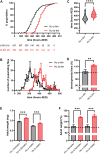
Circadian clocks orchestrate a variety of physiological and behavioural functions within the 24-h day. These timekeeping systems have also been implicated in developmental and reproductive processes that span more (or less) than 24 h. Whether natural alleles of cardinal clock genes affect entire sets of life-history traits (i.e., reproductive arrest, developmental time, fecundity), thus providing a wider substrate for seasonal adaptation, remains unclear. Here we show that natural alleles of the timeless (tim) gene of Drosophila melanogaster, previously shown to modulate flies' propensity to enter reproductive dormancy, differentially affect correlated traits such as early-life fecundity and developmental time. Homozygous flies expressing the shorter TIM isoform (encoded by the s-tim allele) not only show a lower dormancy incidence compared to those homozygous for ls-tim (which produce both the short and an N-terminal additional 23-residues longer TIM isoform), but also higher fecundity in the first 12 days of adult life. Moreover, s-tim homozygous flies develop faster than ls-tim homozygous flies at both warm (25°C) and cold (15°C) temperatures, with the gap being larger at 15°C. In summary, this phenotypic analysis shows that natural variants of tim affect a set of life-history traits associated with reproductive dormancy in Drosophila. We speculate that this provides further adaptive advantage in temperate regions (with seasonal changes) and propose that the underlying mechanisms might not be exclusively dependent on photoperiod, as previously suggested.
Keywords: circadian clock; developmental time; early-life fecundity; photoperiodism; reproductive dormancy; seasonality; timeless.
Copyright © 2023 Andreatta, Montagnese and Costa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Adaptation of Drosophila melanogaster to Long Photoperiods of High-Latitude Summers Is Facilitated by the ls-Timeless Allele.J Biol Rhythms. 2022 Apr;37(2):185-201. doi: 10.1177/07487304221082448. Epub 2022 Mar 18. J Biol Rhythms. 2022. PMID: 35301885 Free PMC article.
-
Clock-gated photic stimulation of timeless expression at cold temperatures and seasonal adaptation in Drosophila.J Biol Rhythms. 2006 Aug;21(4):256-71. doi: 10.1177/0748730406289306. J Biol Rhythms. 2006. PMID: 16864646
-
EYES ABSENT and TIMELESS integrate photoperiodic and temperature cues to regulate seasonal physiology in Drosophila.Proc Natl Acad Sci U S A. 2020 Jun 30;117(26):15293-15304. doi: 10.1073/pnas.2004262117. Epub 2020 Jun 15. Proc Natl Acad Sci U S A. 2020. PMID: 32541062 Free PMC article.
-
Timeless in animal circadian clocks and beyond.FEBS J. 2022 Nov;289(21):6559-6575. doi: 10.1111/febs.16253. Epub 2021 Nov 18. FEBS J. 2022. PMID: 34699674 Free PMC article. Review.
-
Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases.Chronobiol Int. 2002 Sep;19(5):807-64. doi: 10.1081/cbi-120014569. Chronobiol Int. 2002. PMID: 12405549 Review.
Cited by
-
Cold-Sensing TRP Channels and Temperature Preference Modulate Ovarian Development in the Model Organism Drosophila melanogaster.Int J Mol Sci. 2025 Jun 12;26(12):5638. doi: 10.3390/ijms26125638. Int J Mol Sci. 2025. PMID: 40565102 Free PMC article.
-
Integration of photoperiodic and temperature cues by the circadian clock to regulate insect seasonal adaptations.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):585-599. doi: 10.1007/s00359-023-01667-1. Epub 2023 Aug 16. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37584703 Free PMC article. Review.
-
Clock-talk: have we forgotten about geographic variation?J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):649-666. doi: 10.1007/s00359-023-01643-9. Epub 2023 Jun 16. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37322375 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

