Effect of early postnatal supplementation of newborns with probiotic strain E. coli O83:K24:H31 on allergy incidence, dendritic cells, and microbiota
- PMID: 36703968
- PMCID: PMC9872645
- DOI: 10.3389/fimmu.2022.1038328
Effect of early postnatal supplementation of newborns with probiotic strain E. coli O83:K24:H31 on allergy incidence, dendritic cells, and microbiota
Abstract
Introduction: Probiotic administration seems to be a rational approach to promote maturation of the neonatal immune system. Mutual interaction of the microbiota with the host immune system is critical for the setting of appropriate immune responses including a tolerogenic one and thevmaintenance of homeostasis. On the other hand, our knowledge on the modes of actions of probiotics is still scarce.
Methods: In our study, probiotic strain Escherichia coli O83:K24:H31 (EcO83) was administered to neonates of allergic mothers (AMs; neonates with increased risk for allergy development) within 48 h after the delivery, and the impact of this early postnatal supplementation on allergy incidence and selected immune markers has been analyzed 10 years after the primary EcO83 administration.
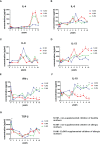
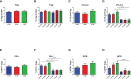
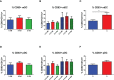
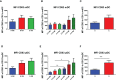
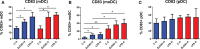
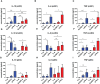
Results: We have observed decreased allergy incidence in 10-year-old children supplemented with EcO83 (13 of 52 children were allergic) in comparison with non-supplemented children of AMs (16 of 42 children were allergic). The early postnatal EcO83 supplementation appeared to limit the allergy in the high-risk group (children of AMs) compared to that in the low-risk group (children of healthy mothers). Dendritic cells (DCs) in the peripheral blood of EcO83-supplemented children do not differ significantly in cell surface presence of CD83. The immunomodulatory capacity of EcO83 on DCs was tested in vitro as well. Both directly isolated myeloid and in vitro monocyte-derived DCs from cord blood increased CD83 expression together with interleukin (IL)-10 secretion after EcO83 stimulation. The effect of early postnatal EcO83 supplementation on the microbiota composition of 10-year-old children was characterized by next-generation sequencing, and we have not observed significant changes in the microbiota composition of EcO83-supplemented and non-supplemented children at the age of 10 years.
Conclusions: Early postnatal EcO83 supplementation appears to lower allergy incidence in children of AMs. It seems that the beneficial effect of EcO83 is mediated via modulation of DC functional capacities without impacting the microbiota composition. Larger-scale studies will be necessary to confirm these preliminary findings.
Keywords: CD83; Escherichia coli O83:K24:H31; IL-10; allergy; cord blood; dendritic cell; flow cytometry; probiotic.
Copyright © 2023 Súkeníková, Černý, Thon, Roubalová, Jirásková Zákostelská, Novotná, Petrásková, Boráková, Kocourková, Lodinová-Žádníková, Musil, Kolářová, Prokešová, Valenta and Hrdý.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Decreased allergy incidence in children supplemented with E. coli O83:K24:H31 and its possible modes of action.Eur J Immunol. 2018 Dec;48(12):2015-2030. doi: 10.1002/eji.201847636. Epub 2018 Oct 21. Eur J Immunol. 2018. PMID: 30306557
-
The Impact of Escherichia coli Probiotic Strain O83:K24:H31 on the Maturation of Dendritic Cells and Immunoregulatory Functions In Vitro and In Vivo.Cells. 2022 May 12;11(10):1624. doi: 10.3390/cells11101624. Cells. 2022. PMID: 35626660 Free PMC article.
-
Different capacity of in vitro generated myeloid dendritic cells of newborns of healthy and allergic mothers to respond to probiotic strain E. coli O83:K24:H31.Immunol Lett. 2017 Sep;189:82-89. doi: 10.1016/j.imlet.2017.05.013. Epub 2017 May 26. Immunol Lett. 2017. PMID: 28554713
-
Probiotics: use in allergic disorders: a Nutrition, Allergy, Mucosal Immunology, and Intestinal Microbiota (NAMI) Research Group Report.J Clin Gastroenterol. 2008 Jul;42 Suppl 2:S91-6. doi: 10.1097/MCG.0b013e3181639a98. J Clin Gastroenterol. 2008. PMID: 18542035 Review.
-
Low-grade disease activity in early life precedes childhood asthma and allergy.Dan Med J. 2016 Aug;63(8):B5272. Dan Med J. 2016. PMID: 27477800 Review.
Cited by
-
Bacterial extracellular vesicles as intranasal postbiotics: Detailed characterization and interaction with airway cells.J Extracell Vesicles. 2024 Oct;13(10):e70004. doi: 10.1002/jev2.70004. J Extracell Vesicles. 2024. PMID: 39429019 Free PMC article.
References
-
- Lohonková A, Novotná O, Petrásková P, Boráková K, Prokešová L, Hrdý J. Maternal allergy status has no impact on neonatal immune responses to allergen stimuli. Folia Biol (2019) 65:221–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

