Melanocortin receptor agonist NDP-α-MSH improves cognitive deficits and microgliosis but not amyloidosis in advanced stages of AD progression in 5XFAD and 3xTg mice
- PMID: 36703981
- PMCID: PMC9871936
- DOI: 10.3389/fimmu.2022.1082036
Melanocortin receptor agonist NDP-α-MSH improves cognitive deficits and microgliosis but not amyloidosis in advanced stages of AD progression in 5XFAD and 3xTg mice
Abstract
Introduction: Alzheimer's disease (AD) is the most frequent cause of dementia and still lacks effective therapy. Clinical signs of AD include low levels of endogenous melanocortins (MCs) and previous studies have shown that treatment with MC analogs induces neuroprotection in the early stages of AD.
Methods: We investigated the neuroprotective role of MCs in two transgenic mouse models of severe AD using 5 and 7 month-old (mo) 5XFAD mice and 9 and 12 mo 3xTg mice. These mice were subjected to a chronic stimulation of MC receptors (MCRs) with MC analogue Nle4-D-Phe7-α-melanocyte stimulating hormone (NDP-α-MSH, 340 μg/kg, i.p.). Mouse behavior and ex-vivo histological and biochemical analyses were performed after 50 days of treatment.
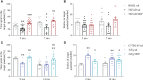
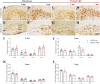
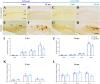
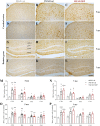
Results: Our analysis demonstrated an improvement in cognitive abilities of AD mice at late stage of AD progression. We also showed that these protective effects are associated with decreased levels of hyperphosphorylated Tau but not with Aβ burden, that was unaffected in the hippocampus and in the cortex of AD mice. In addition, an age-dependent NDP effect on glial reactivity was observed only in 3xTg mice whereas a global downregulation of p38 mitogen-activated protein kinase was selectively observed in 7 mo 5XFAD and 14 mo 3xTg mice.
Conclusion: Our results suggest that MCR stimulation by NDP-α-MSH could represent a promising therapeutic strategy in managing cognitive decline also at late stage of AD, whereas the effects on neuroinflammation may be restricted to specific stages of AD progression.
Keywords: 3xTg; 5XFAD; NDP-α-MSH; cognitive deficits; neuroprotection.
Copyright © 2023 Daini, Vandini, Bodria, Liao, Baraldi, Secco, Ottani, Zoli, Giuliani and Vilella.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures



Similar articles
-
NDP-α-MSH induces intense neurogenesis and cognitive recovery in Alzheimer transgenic mice through activation of melanocortin MC4 receptors.Mol Cell Neurosci. 2015 Jul;67:13-21. doi: 10.1016/j.mcn.2015.05.004. Epub 2015 May 21. Mol Cell Neurosci. 2015. PMID: 26003413
-
Melanocortins protect against progression of Alzheimer's disease in triple-transgenic mice by targeting multiple pathophysiological pathways.Neurobiol Aging. 2014 Mar;35(3):537-47. doi: 10.1016/j.neurobiolaging.2013.08.030. Epub 2013 Oct 1. Neurobiol Aging. 2014. PMID: 24094579
-
Melanocortins protect against brain damage and counteract cognitive decline in a transgenic mouse model of moderate Alzheimer׳s disease.Eur J Pharmacol. 2014 Oct 5;740:144-50. doi: 10.1016/j.ejphar.2014.06.063. Epub 2014 Jul 15. Eur J Pharmacol. 2014. PMID: 25034807
-
Combined effects of aerobic exercise and 40-Hz light flicker exposure on early cognitive impairments in Alzheimer's disease of 3×Tg mice.J Appl Physiol (1985). 2022 Apr 1;132(4):1054-1068. doi: 10.1152/japplphysiol.00751.2021. Epub 2022 Feb 24. J Appl Physiol (1985). 2022. PMID: 35201933
-
α-Melanocyte Stimulating Hormone as a Potential Therapy for Alzheimer`s Disease.Curr Alzheimer Res. 2017;14(1):18-29. doi: 10.2174/1567205013666160819130641. Curr Alzheimer Res. 2017. PMID: 27539595 Review.
Cited by
-
Evidence for a protective effect of the loss of α4-containing nicotinic acetylcholine receptors on Aβ-related neuropathology in Tg2576 mice.Front Neurosci. 2023 Apr 11;17:1097857. doi: 10.3389/fnins.2023.1097857. eCollection 2023. Front Neurosci. 2023. PMID: 37113156 Free PMC article.
-
Anorexigenic neuropeptides as anti-obesity and neuroprotective agents: exploring the neuroprotective effects of anorexigenic neuropeptides.Biosci Rep. 2024 Apr 24;44(4):BSR20231385. doi: 10.1042/BSR20231385. Biosci Rep. 2024. PMID: 38577975 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

