Alpha-1 antitrypsin limits neutrophil extracellular trap disruption of airway epithelial barrier function
- PMID: 36703990
- PMCID: PMC9872031
- DOI: 10.3389/fimmu.2022.1023553
Alpha-1 antitrypsin limits neutrophil extracellular trap disruption of airway epithelial barrier function
Abstract
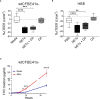
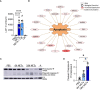
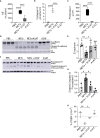
Neutrophil extracellular traps contribute to lung injury in cystic fibrosis and asthma, but the mechanisms are poorly understood. We sought to understand the impact of human NETs on barrier function in primary human bronchial epithelial and a human airway epithelial cell line. We demonstrate that NETs disrupt airway epithelial barrier function by decreasing transepithelial electrical resistance and increasing paracellular flux, partially by NET-induced airway cell apoptosis. NETs selectively impact the expression of tight junction genes claudins 4, 8 and 11. Bronchial epithelia exposed to NETs demonstrate visible gaps in E-cadherin staining, a decrease in full-length E-cadherin protein and the appearance of cleaved E-cadherin peptides. Pretreatment of NETs with alpha-1 antitrypsin (A1AT) inhibits NET serine protease activity, limits E-cadherin cleavage, decreases bronchial cell apoptosis and preserves epithelial integrity. In conclusion, NETs disrupt human airway epithelial barrier function through bronchial cell death and degradation of E-cadherin, which are limited by exogenous A1AT.
Keywords: E-cadherin (CDH1); NETs (neutrophil extracellular traps); alpha-1 antitrypsin (A1AT); barrier function; bronchial epithelia.
Copyright © 2023 Hudock, Collins, Imbrogno, Kramer, Brewington, Ziady, Zhang, Snowball, Xu, Carey, Horio, O’Grady, Kopras, Meeker, Morgan, Ostmann, Skala, Siefert, Na, Davidson, Gollomp, Mangalmurti, Trapnell and Clancy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Neutrophil extracellular traps activate IL-8 and IL-1 expression in human bronchial epithelia.Am J Physiol Lung Cell Mol Physiol. 2020 Jul 1;319(1):L137-L147. doi: 10.1152/ajplung.00144.2019. Epub 2020 Mar 11. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32159969 Free PMC article.
-
Blockade of neutrophil extracellular traps ameliorates toluene diisocyanate-induced steroid-resistant asthma.Int Immunopharmacol. 2023 Apr;117:109719. doi: 10.1016/j.intimp.2023.109719. Epub 2023 Feb 22. Int Immunopharmacol. 2023. PMID: 36827917
-
Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma.Clin Exp Allergy. 2017 Jan;47(1):57-70. doi: 10.1111/cea.12859. Clin Exp Allergy. 2017. PMID: 27883241
-
Progression of Cystic Fibrosis Lung Disease from Childhood to Adulthood: Neutrophils, Neutrophil Extracellular Trap (NET) Formation, and NET Degradation.Genes (Basel). 2019 Feb 26;10(3):183. doi: 10.3390/genes10030183. Genes (Basel). 2019. PMID: 30813645 Free PMC article. Review.
-
NETopathic Inflammation in Chronic Obstructive Pulmonary Disease and Severe Asthma.Front Immunol. 2019 Feb 5;10:47. doi: 10.3389/fimmu.2019.00047. eCollection 2019. Front Immunol. 2019. PMID: 30804927 Free PMC article. Review.
Cited by
-
Heterogeneity in Neutrophil Extracellular Traps from Healthy Human Subjects.Int J Mol Sci. 2023 Dec 30;25(1):525. doi: 10.3390/ijms25010525. Int J Mol Sci. 2023. PMID: 38203698 Free PMC article.
-
Pseudomonas aeruginosa in chronic lung disease: untangling the dysregulated host immune response.Front Immunol. 2024 Jun 28;15:1405376. doi: 10.3389/fimmu.2024.1405376. eCollection 2024. Front Immunol. 2024. PMID: 39015565 Free PMC article. Review.
-
The role of extracellular traps released by neutrophils, eosinophils, and macrophages in asthma.Respir Res. 2024 Jul 30;25(1):290. doi: 10.1186/s12931-024-02923-x. Respir Res. 2024. PMID: 39080638 Free PMC article. Review.
-
Multifunctional Boron-based 2D Nanoplatforms Ameliorate Severe Respiratory Inflammation by Targeting Multiple Inflammatory Mediators.Adv Sci (Weinh). 2025 Apr;12(13):e2412626. doi: 10.1002/advs.202412626. Epub 2025 Feb 14. Adv Sci (Weinh). 2025. PMID: 39950864 Free PMC article.
-
Synthetic neutrophil extracellular traps dissect bactericidal contribution of NETs under regulation of α-1-antitrypsin.Sci Adv. 2023 Apr 28;9(17):eadf2445. doi: 10.1126/sciadv.adf2445. Epub 2023 Apr 28. Sci Adv. 2023. PMID: 37115934 Free PMC article.
References
-
- Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, et al. . Neutrophil extracellular trap (NET)-mediated killing of pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PloS One (2011) 6(9):e23637. doi: 10.1371/journal.pone.0023637 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

