Generation of bispecific antibodies by structure-guided redesign of IgG constant regions
- PMID: 36703993
- PMCID: PMC9871890
- DOI: 10.3389/fimmu.2022.1063002
Generation of bispecific antibodies by structure-guided redesign of IgG constant regions
Abstract
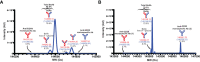
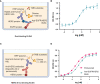
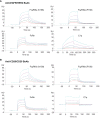
Bispecific antibodies (BsAbs) form an exciting class of bio-therapeutics owing to their multispecificity. Although numerous formats have been developed, generation of hetero-tetrameric IgG1-like BsAbs having acceptable safety and pharmacokinetics profiles from a single cell culture system remains challenging due to the heterogeneous pairing between the four chains. Herein, we employed a structure-guided approach to engineer mutations in the constant domain interfaces (CH1-CL and CH3-CH3) of heavy and κ light chains to prevent heavy-light mispairing in the antigen binding fragment (Fab) region and heavy-heavy homodimerization in the Fc region. Transient co-transfection of mammalian cells with heavy and light chains of pre-existing antibodies carrying the engineered constant domains generates BsAbs with percentage purity ranging from 78% to 85%. The engineered BsAbs demonstrate simultaneous binding of both antigens, while retaining the thermal stability, Fc-mediated effector properties and FcRn binding properties of the parental antibodies. Importantly, since the variable domains were not modified, the mutations may enable BsAb formation from antibodies belonging to different germline origins and isotypes. The rationally designed mutations reported in this work could serve as a starting point for generating optimized solutions required for large scale production.
Keywords: EGFR – epidermal growth factor; Fc engineering; Fc receptors (FcR); HER2; bispecific antibody (BsAb); heterodimer; rational design; structure-guided.
Copyright © 2023 Iwasaki, Tharakaraman, Subramanian, Khongmanee, Hatas, Fleischer, Rurak, Ngok-ngam, Tit-oon, Ruchirawat, Satayavivad, Fuangthong and Sasisekharan.
Conflict of interest statement
The mutations described in the manuscript are the subject of a patent application by Massachusetts Institute of Technology on which KT, VS, AH, RS are inventors. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Xu J, Stanislaus S, Chinookoswong N, Lau YY, Hager T, Patel J, et al. . Acute glucose-lowering and insulin-sensitizing action of Fgf21 in insulin-resistant mouse models–association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab (2009) 297(5):E1105–E14. doi: 10.1152/ajpendo.00348.2009 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

