A dual-flow RootChip enables quantification of bi-directional calcium signaling in primary roots
- PMID: 36704158
- PMCID: PMC9871814
- DOI: 10.3389/fpls.2022.1040117
A dual-flow RootChip enables quantification of bi-directional calcium signaling in primary roots
Abstract
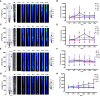
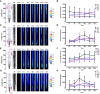
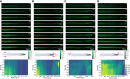
One sentence summary: Bi-directional-dual-flow-RootChip to track calcium signatures in Arabidopsis primary roots responding to osmotic stress. Plant growth and survival is fundamentally linked with the ability to detect and respond to abiotic and biotic factors. Cytosolic free calcium (Ca2+) is a key messenger in signal transduction pathways associated with a variety of stresses, including mechanical, osmotic stress and the plants' innate immune system. These stresses trigger an increase in cytosolic Ca2+ and thus initiate a signal transduction cascade, contributing to plant stress adaptation. Here we combine fluorescent G-CaMP3 Arabidopsis thaliana sensor lines to visualise Ca2+ signals in the primary root of 9-day old plants with an optimised dual-flow RootChip (dfRC). The enhanced polydimethylsiloxane (PDMS) bi-directional-dual-flow-RootChip (bi-dfRC) reported here adds two adjacent inlet channels at the base of the observation chamber, allowing independent or asymmetric chemical stimulation at either the root differentiation zone or tip. Observations confirm distinct early spatio-temporal patterns of salinity (sodium chloride, NaCl) and drought (polyethylene glycol, PEG)-induced Ca2+ signals throughout different cell types dependent on the first contact site. Furthermore, we show that the primary signal always dissociates away from initially stimulated cells. The observed early signaling events induced by NaCl and PEG are surprisingly complex and differ from long-term changes in cytosolic Ca2+ reported in roots. Bi-dfRC microfluidic devices will provide a novel approach to challenge plant roots with different conditions simultaneously, while observing bi-directionality of signals. Future applications include combining the bi-dfRC with H2O2 and redox sensor lines to test root systemic signaling responses to biotic and abiotic factors.
Keywords: Arabidopsis; abiotic stress; calcium; microfluidics; osmotic stress; root; signalling.
Copyright © 2023 Allan, Tayagui, Hornung, Nock and Meisrimler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Observing root growth and signalling responses to stress gradients and pathogens using the bi-directional dual-flow RootChip.Lab Chip. 2024 Dec 3;24(24):5360-5373. doi: 10.1039/d4lc00659c. Lab Chip. 2024. PMID: 39508314 Free PMC article.
-
Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments.Bio Protoc. 2023 Aug 5;13(15):e4764. doi: 10.21769/BioProtoc.4764. eCollection 2023 Aug 5. Bio Protoc. 2023. PMID: 37575387 Free PMC article.
-
Dual-flow-RootChip reveals local adaptations of roots towards environmental asymmetry at the physiological and genetic levels.New Phytol. 2018 Feb;217(3):1357-1369. doi: 10.1111/nph.14887. Epub 2017 Nov 10. New Phytol. 2018. PMID: 29125191
-
Crosstalk between Ca2+ and Other Regulators Assists Plants in Responding to Abiotic Stress.Plants (Basel). 2022 May 19;11(10):1351. doi: 10.3390/plants11101351. Plants (Basel). 2022. PMID: 35631776 Free PMC article. Review.
-
Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress.Adv Exp Med Biol. 2018;1081:215-232. doi: 10.1007/978-981-13-1244-1_12. Adv Exp Med Biol. 2018. PMID: 30288712 Review.
Cited by
-
Counter-on-chip for bacterial cell quantification, growth, and live-dead estimations.Sci Rep. 2024 Jan 8;14(1):782. doi: 10.1038/s41598-023-51014-2. Sci Rep. 2024. PMID: 38191788 Free PMC article.
-
Observing root growth and signalling responses to stress gradients and pathogens using the bi-directional dual-flow RootChip.Lab Chip. 2024 Dec 3;24(24):5360-5373. doi: 10.1039/d4lc00659c. Lab Chip. 2024. PMID: 39508314 Free PMC article.
-
Bi-directional Dual-flow-RootChip for Physiological Analysis of Plant Primary Roots Under Asymmetric Perfusion of Stress Treatments.Bio Protoc. 2023 Aug 5;13(15):e4764. doi: 10.21769/BioProtoc.4764. eCollection 2023 Aug 5. Bio Protoc. 2023. PMID: 37575387 Free PMC article.
References
-
- Allan C. (2021). Implementation of a novel bi-directional dual-flow-RootChip to investigate the effects of osmotic stress on calcium signalling in Arabidopsis thaliana roots. (University of Canterbury MSc thesis; ). doi: 10.26021/11069 - DOI
-
- Allan C., Tayagui A., Nock V., Meisrimler C. N. (2022. b). “Novel bi-directional dual-flow-RootChip to study effects of osmotic stress on calcium signalling in Arabidopsis roots,” in 2022 IEEE 35th International Conference on Micro Electro Mechanical Systems Conference (MEMS), 2022, 896–899. doi: 10.1109/MEMS51670.2022.9699700 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

