Plausible Sources of Membrane-Forming Fatty Acids on the Early Earth: A Review of the Literature and an Estimation of Amounts
- PMID: 36704178
- PMCID: PMC9869395
- DOI: 10.1021/acsearthspacechem.2c00168
Plausible Sources of Membrane-Forming Fatty Acids on the Early Earth: A Review of the Literature and an Estimation of Amounts
Abstract
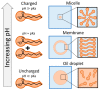
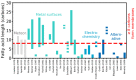
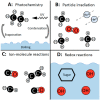
The first cells were plausibly bounded by membranes assembled from fatty acids with at least 8 carbons. Although the presence of fatty acids on the early Earth is widely assumed within the astrobiology community, there is no consensus regarding their origin and abundance. In this Review, we highlight three possible sources of fatty acids: (1) delivery by carbonaceous meteorites, (2) synthesis on metals delivered by impactors, and (3) electrochemical synthesis by spark discharges. We also discuss fatty acid synthesis by UV or particle irradiation, gas-phase ion-molecule reactions, and aqueous redox reactions. We compare estimates for the total mass of fatty acids supplied to Earth by each source during the Hadean eon after an extremely massive asteroid impact that would have reset Earth's fatty acid inventory. We find that synthesis on iron-rich surfaces derived from the massive impactor in contact with an impact-generated reducing atmosphere could have contributed ∼102 times more total mass of fatty acids than subsequent delivery by either carbonaceous meteorites or electrochemical synthesis. Additionally, we estimate that a single carbonaceous meteorite would not deliver a high enough concentration of fatty acids (∼15 mM for decanoic acid) into an existing body of water on the Earth's surface to spontaneously form membranes unless the fatty acids were further concentrated by another mechanism, such as subsequent evaporation of the water. Our estimates rely heavily on various assumptions, leading to significant uncertainties; nevertheless, these estimates provide rough order-of-magnitude comparisons of various sources of fatty acids on the early Earth. We also suggest specific experiments to improve future estimates. Our calculations support the view that fatty acids would have been available on the early Earth. Further investigation is needed to assess the mechanisms by which fatty acids could have been concentrated sufficiently to assemble into membranes during the origin of life.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Accretion and differentiation of carbon in the early Earth.Chem Geol. 1998 May 15;147(1-2):3-10. doi: 10.1016/s0009-2541(97)00168-x. Chem Geol. 1998. PMID: 11543125
-
Formation of Amino Acids and Carboxylic Acids in Weakly Reducing Planetary Atmospheres by Solar Energetic Particles from the Young Sun.Life (Basel). 2023 Apr 28;13(5):1103. doi: 10.3390/life13051103. Life (Basel). 2023. PMID: 37240748 Free PMC article.
-
Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites.Chem Soc Rev. 2012 Aug 21;41(16):5459-72. doi: 10.1039/c2cs35109a. Epub 2012 Jun 15. Chem Soc Rev. 2012. PMID: 22706603 Review.
-
Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth.Nature. 2001 Dec 20-27;414(6866):879-83. doi: 10.1038/414879a. Nature. 2001. PMID: 11780054
-
Monosaccharides and Their Derivatives in Carbonaceous Meteorites: A Scenario for Their Synthesis and Onset of Enantiomeric Excesses.Life (Basel). 2018 Aug 27;8(3):36. doi: 10.3390/life8030036. Life (Basel). 2018. PMID: 30150578 Free PMC article. Review.
Cited by
-
Evidence of Heritability in Prebiotically Realistic Membrane-Bound Systems.Life (Basel). 2024 Feb 20;14(3):284. doi: 10.3390/life14030284. Life (Basel). 2024. PMID: 38541610 Free PMC article.
-
Information Gradient among Nucleotide Sequences of Essential RNAs from an Evolutionary Perspective.Int J Mol Sci. 2024 Jul 9;25(14):7521. doi: 10.3390/ijms25147521. Int J Mol Sci. 2024. PMID: 39062761 Free PMC article.
-
Optimization and Enhancement of the Peroxidase-like Activity of Hemin in Aqueous Solutions of Sodium Dodecylsulfate.ACS Omega. 2023 Nov 3;8(45):42878-42899. doi: 10.1021/acsomega.3c05915. eCollection 2023 Nov 14. ACS Omega. 2023. PMID: 38024761 Free PMC article.
-
Growth of Prebiotically Plausible Fatty Acid Vesicles Proceeds in the Presence of Prebiotic Amino Acids, Dipeptides, Sugars, and Nucleic Acid Components.Langmuir. 2022 Dec 13;38(49):15106-15112. doi: 10.1021/acs.langmuir.2c02118. Epub 2022 Nov 29. Langmuir. 2022. PMID: 36445982 Free PMC article.
-
Natural soda lakes provide compatible conditions for RNA and membrane function that could have enabled the origin of life.PNAS Nexus. 2024 Mar 19;3(3):pgae084. doi: 10.1093/pnasnexus/pgae084. eCollection 2024 Mar. PNAS Nexus. 2024. PMID: 38505692 Free PMC article.
References
-
- Black R. A.; Blosser M. C.; Stottrup B. L.; Tavakley R.; Deamer D. W.; Keller S. L. Nucleobases Bind to and Stabilize Aggregates of a Prebiotic Amphiphile, Providing a Viable Mechanism for the Emergence of Protocells. Proc. Natl. Acad. Sci. USA 2013, 110 (33), 13272.10.1073/pnas.1300963110. - DOI - PMC - PubMed
-
- Cornell C. E.; Black R. A.; Xue M.; Litz H. E.; Ramsay A.; Gordon M.; Mileant A.; Cohen Z. R.; Williams J. A.; Lee K. K.; Drobny G. P.; Keller S. L. Prebiotic Amino Acids Bind to and Stabilize Prebiotic Fatty Acid Membranes. Proc. Natl. Acad. Sci. USA 2019, 116 (35), 17239–17244. 10.1073/pnas.1900275116. - DOI - PMC - PubMed
-
- Xue M.; Black R. A.; Cohen Z. R.; Roehrich A.; Drobny G. P.; Keller S. L. Binding of Dipeptides to Fatty Acid Membranes Explains Their Colocalization in Protocells but Does Not Select for Them Relative to Unjoined Amino Acids. J. Phys. Chem. B 2021, 125 (29), 7933–7939. 10.1021/acs.jpcb.1c01485. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
