Long-term prognosis of 35 patients with methionine adenosyltransferase deficiency based on newborn screening in China
- PMID: 36704196
- PMCID: PMC9871361
- DOI: 10.3389/fcell.2022.1059680
Long-term prognosis of 35 patients with methionine adenosyltransferase deficiency based on newborn screening in China
Abstract
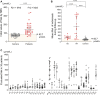
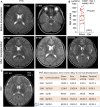
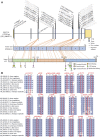
Methionine adenosyltransferase deficiency (MATD) is a rare metabolic disorder caused by mono- or biallelic MAT1A mutations that are not yet well understood. Of the 4,065,644 neonates screened between November 2010 and December 2021, 35 individuals have been diagnosed with an estimated incidence of 1: 116,161 by a cutoff value of methionine 82.7 μmol/L and follow-up over 11 years. MATD patients with autosomal recessive (AR) type had higher clinical and genetic heterogeneity than those with autosomal dominant (AD) type. Fifteen unrelated AD patients harbored one well-known dominant variant, c.791 G>A or c.776 C>T, and were clinically unaffected with a mean plasma methionine (Met) value <300 μmol/L. Twenty AR cases have unique genotypes and presented a wide range of clinical abnormalities from asymptomatic to white matter lesions. Of them, 10 AR patients displayed severe manifestations, such as verbal difficulty, motor delay, development delay, and white matter lesions, with mean Met >500 μmol/L and thereby were treated with a methionine-restricted diet alone or in combination with betaine, folate, or vitamin B6, and were healthy finally. Neurological abnormalities were evidenced in two patients (P16 and P27) with Met values >800 μmol/L by MRI scan. Neurological abnormalities were reversed here by liver transplantation or by the determination of S-adenosylmethionine supplementation. Additionally, 38 variants of MAT1A were distributed within patients and carriers, of which 24 were novel and mostly predicted to be damaged. Our findings with an extensive clinical and genetic dataset provided new insights into its diagnosis and treatment and will be helpful for its optimal management in the future.
Keywords: MAT1A; S-adenosylmethionine; hypermethioninemia; long-term prognosis; methionine adenosyltransferase deficiency (MATD); neurological deficits.
Copyright © 2023 Tong, Zhang, Chen, Zhu, Lu, Zhang, Chen, Yan, Zheng, Zhao, Zhou, Yang, Yang, Cang, Jiang and Shu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Clinical and metabolic findings in patients with methionine adenosyltransferase I/III deficiency detected by newborn screening.Mol Genet Metab. 2013 Nov;110(3):218-21. doi: 10.1016/j.ymgme.2013.08.003. Epub 2013 Aug 14. Mol Genet Metab. 2013. PMID: 23993429
-
Determination of Autosomal Dominant or Recessive Methionine Adenosyltransferase I/III Deficiencies Based on Clinical and Molecular Studies.Mol Med. 2016 Sep;22:147-155. doi: 10.2119/molmed.2015.00254. Epub 2016 Feb 18. Mol Med. 2016. PMID: 26933843 Free PMC article.
-
Methionine Adenosyltransferase I/III Deficiency Detected by Newborn Screening.Genes (Basel). 2022 Jun 27;13(7):1163. doi: 10.3390/genes13071163. Genes (Basel). 2022. PMID: 35885946 Free PMC article.
-
Molecular genetics of hepatic methionine adenosyltransferase deficiency.Pharmacol Ther. 2000 Jan;85(1):1-9. doi: 10.1016/s0163-7258(99)00047-9. Pharmacol Ther. 2000. PMID: 10674710 Review.
-
Methionine adenosyltransferase I/III deficiency: neurological manifestations and relevance of S-adenosylmethionine.Mol Genet Metab. 2012 Nov;107(3):253-6. doi: 10.1016/j.ymgme.2012.08.002. Epub 2012 Aug 11. Mol Genet Metab. 2012. PMID: 22951388 Review.
Cited by
-
Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020-2023).Int J Neonatal Screen. 2024 May 23;10(2):38. doi: 10.3390/ijns10020038. Int J Neonatal Screen. 2024. PMID: 38920845 Free PMC article. Review.
-
Genetic variation and clinical phenotype analysis of hypermethioninemia caused by MAT1A gene mutation: Case report.Medicine (Baltimore). 2024 Dec 20;103(51):e40957. doi: 10.1097/MD.0000000000040957. Medicine (Baltimore). 2024. PMID: 39705457 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

