Precipitated opioid withdrawal after buprenorphine administration in patients presenting to the emergency department: A case series
- PMID: 36704210
- PMCID: PMC9871399
- DOI: 10.1002/emp2.12880
Precipitated opioid withdrawal after buprenorphine administration in patients presenting to the emergency department: A case series
Abstract
Objectives: Buprenorphine is a highly effective medication for the treatment of opioid use disorder, but it can cause precipitated withdrawal (PW) from opioids. Incidence, risk factors, and best approaches to management of PW are not well understood. Our objective was to describe adverse outcomes after buprenorphine administration among emergency department (ED) patients and assess whether they met the criteria for PW.
Methods: This study is a case series using retrospective chart review in a convenience sample of patients from 3 hospitals in an urban academic health system. This study included patients who were reported by clinicians as potential cases of PW. Relevant clinical data were abstracted from the electronic health record using a structured retrospective chart review instrument.
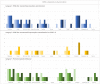
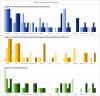
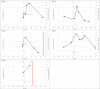
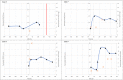
Results: A total of 13 cases were included and classified into the following 3 categories: (1) PW after buprenorphine administration consistent with guidelines (n = 5), (2) PW after deviating from guidelines (n = 4), and (3) protracted opioid withdrawal with no increase in Clinical Opiate Withdrawal Scale score (n = 4). A total of 11 patients had urine drug testing positive for fentanyl, and 11 patients received additional doses of buprenorphine for symptom management. Of the patients, 5 had self-directed hospital discharges, and 6 were ultimately discharged with prescriptions for buprenorphine.
Conclusions: Cases of adverse outcomes after buprenorphine administration in the ED and hospital meet criteria for PW, although some cases may have represented protracted opioid withdrawal. Further investigation into the incidence, risk factors, management of PW as well as patient perspectives is needed to expand and sustain the use of buprenorphine in EDs and hospitals.
© 2023 The Authors. JACEP Open published by Wiley Periodicals LLC on behalf of American College of Emergency Physicians.
Conflict of interest statement
None.
Figures
Similar articles
-
Buprenorphine precipitated opioid withdrawal: Prevention and management in the ED setting.Am J Emerg Med. 2022 Aug;58:22-26. doi: 10.1016/j.ajem.2022.05.013. Epub 2022 May 14. Am J Emerg Med. 2022. PMID: 35623179 Review.
-
Buprenorphine for Opioid Use Disorder in the Emergency Department: A Retrospective Chart Review.West J Emerg Med. 2020 Aug 24;21(5):1175-1181. doi: 10.5811/westjem.2020.6.46452. West J Emerg Med. 2020. PMID: 32970572 Free PMC article.
-
Treatment of acute naloxone-precipitated opioid withdrawal with buprenorphine.Am J Emerg Med. 2020 Mar;38(3):691.e3-691.e4. doi: 10.1016/j.ajem.2019.09.014. Epub 2019 Nov 16. Am J Emerg Med. 2020. PMID: 31753622
-
Transdermal buprenorphine for in-hospital transition from full agonist opioids to sublingual buprenorphine: a retrospective observational cohort study.Clin Toxicol (Phila). 2022 Jun;60(6):688-693. doi: 10.1080/15563650.2022.2028802. Epub 2022 Jan 20. Clin Toxicol (Phila). 2022. PMID: 35048759
-
Approach to buprenorphine use for opioid withdrawal treatment in the emergency setting.Am J Emerg Med. 2019 Jan;37(1):143-150. doi: 10.1016/j.ajem.2018.10.013. Epub 2018 Oct 11. Am J Emerg Med. 2019. PMID: 30355476 Review.
Cited by
-
Extended-Release Injection vs Sublingual Buprenorphine for Opioid Use Disorder With Fentanyl Use: A Post Hoc Analysis of a Randomized Clinical Trial.JAMA Netw Open. 2024 Jun 3;7(6):e2417377. doi: 10.1001/jamanetworkopen.2024.17377. JAMA Netw Open. 2024. PMID: 38916892 Free PMC article. Clinical Trial.
-
Escalating Doses of Buprenorphine for the Treatment of Buprenorphine-Induced Opioid Withdrawal.Cureus. 2025 Mar 13;17(3):e80527. doi: 10.7759/cureus.80527. eCollection 2025 Mar. Cureus. 2025. PMID: 40225488 Free PMC article.
-
Methadone Initiation in the Emergency Department for Opioid Use Disorder.West J Emerg Med. 2024 Sep;25(5):668-674. doi: 10.5811/westjem.18530. West J Emerg Med. 2024. PMID: 39319796 Free PMC article.
-
Things We Do for No Reason™: Avoiding methadone for opioid withdrawal.J Hosp Med. 2023 Nov;18(11):1034-1037. doi: 10.1002/jhm.13138. Epub 2023 May 27. J Hosp Med. 2023. PMID: 37244869 Free PMC article. No abstract available.
-
Buprenorphine-Precipitated Withdrawal Among Hospitalized Patients Using Fentanyl.JAMA Netw Open. 2024 Sep 3;7(9):e2435895. doi: 10.1001/jamanetworkopen.2024.35895. JAMA Netw Open. 2024. PMID: 39331392 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
