The role of the endolysosomal pathway in α-synuclein pathogenesis in Parkinson's disease
- PMID: 36704248
- PMCID: PMC9871505
- DOI: 10.3389/fncel.2022.1081426
The role of the endolysosomal pathway in α-synuclein pathogenesis in Parkinson's disease
Abstract
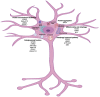
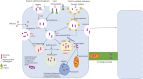
Parkinson's disease (PD) is a chronic neurodegenerative disease that is characterized by a loss of dopaminergic neurons in the substantia nigra pars compacta of the midbrain (SNpc). Extensive studies into genetic and cellular models of PD implicate protein trafficking as a prominent contributor to the death of these dopaminergic neurons. Considerable evidence also suggests the involvement of α-synuclein as a central component of the characteristic cell death in PD and it is a major structural constituent of proteinaceous inclusion bodies (Lewy bodies; LB). α-synuclein research has been a vital part of PD research in recent years, with newly discovered evidence suggesting that α-synuclein can propagate through the brain via prion-like mechanisms. Healthy cells can internalize toxic α-synuclein species and seed endogenous α-synuclein to form large, pathogenic aggregates and form LBs. A better understanding of how α-synuclein can propagate, enter and be cleared from the cell is vital for therapeutic strategies.
Keywords: Parkinson’s disease; endocytosis; endolysosomal; trafficking; α-synuclein.
Copyright © 2023 Smith, Mellick and Sykes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
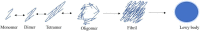
Similar articles
-
The central theme of Parkinson's disease: α-synuclein.Mol Neurobiol. 2013 Apr;47(2):460-5. doi: 10.1007/s12035-012-8369-3. Epub 2012 Nov 23. Mol Neurobiol. 2013. PMID: 23180276 Review.
-
[Role of microglial activation induced by α-synuclein in pathogenesis of Parkinson's disease].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012 Mar;41(2):210-4. doi: 10.3785/j.issn.1008-9292.2012.02.016. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2012. PMID: 22499522 Review. Chinese.
-
Inoculation of α-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve.Mol Neurodegener. 2018 May 11;13(1):21. doi: 10.1186/s13024-018-0257-5. Mol Neurodegener. 2018. PMID: 29751824 Free PMC article.
-
CD8 T cell nigral infiltration precedes synucleinopathy in early stages of Parkinson's disease.Brain. 2020 Dec 1;143(12):3717-3733. doi: 10.1093/brain/awaa269. Brain. 2020. PMID: 33118032
-
Decoding crosstalk between neurotransmitters and α-synuclein in Parkinson's disease: pathogenesis and therapeutic implications.Ther Adv Neurol Disord. 2025 Jun 5;18:17562864251339895. doi: 10.1177/17562864251339895. eCollection 2025. Ther Adv Neurol Disord. 2025. PMID: 40486190 Free PMC article. Review.
Cited by
-
New aspects of a small GTPase RAB35 in brain development and function.Neural Regen Res. 2025 Jul 1;20(7):1971-1980. doi: 10.4103/NRR.NRR-D-23-01543. Epub 2024 Mar 1. Neural Regen Res. 2025. PMID: 39254551 Free PMC article.
-
Is There a Place for Lewy Bodies before and beyond Alpha-Synuclein Accumulation? Provocative Issues in Need of Solid Explanations.Int J Mol Sci. 2024 Apr 1;25(7):3929. doi: 10.3390/ijms25073929. Int J Mol Sci. 2024. PMID: 38612739 Free PMC article. Review.
-
Emerging targets of α-synuclein spreading in α-synucleinopathies: a review of mechanistic pathways and interventions.Mol Neurodegener. 2025 Jan 23;20(1):10. doi: 10.1186/s13024-025-00797-1. Mol Neurodegener. 2025. PMID: 39849529 Free PMC article. Review.
-
In situ stoichiometry amounts of p62 and poly-ubiquitin exceed the increase of alpha-synuclein during degeneration of catecholamine cells induced by autophagy inhibition in vitro.J Neural Transm (Vienna). 2024 Dec;131(12):1397-1414. doi: 10.1007/s00702-024-02795-x. Epub 2024 Jun 18. J Neural Transm (Vienna). 2024. PMID: 38890195 Free PMC article.
-
Plasma Metabolites as Mediators Between Gut Microbiota and Parkinson's Disease: Insights from Mendelian Randomization.Mol Neurobiol. 2025 Jun;62(6):7945-7956. doi: 10.1007/s12035-025-04765-0. Epub 2025 Feb 17. Mol Neurobiol. 2025. PMID: 39962023
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

