The olfactory limbus of the red fox (Vulpes vulpes). New insights regarding a noncanonical olfactory bulb pathway
- PMID: 36704406
- PMCID: PMC9871471
- DOI: 10.3389/fnana.2022.1097467
The olfactory limbus of the red fox (Vulpes vulpes). New insights regarding a noncanonical olfactory bulb pathway
Abstract
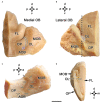
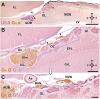
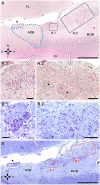
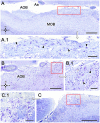
Introduction: The olfactory system in most mammals is divided into several subsystems based on the anatomical locations of the neuroreceptor cells involved and the receptor families that are expressed. In addition to the main olfactory system and the vomeronasal system, a range of olfactory subsystems converge onto the transition zone located between the main olfactory bulb (MOB) and the accessory olfactory bulb (AOB), which has been termed the olfactory limbus (OL). The OL contains specialized glomeruli that receive noncanonical sensory afferences and which interact with the MOB and AOB. Little is known regarding the olfactory subsystems of mammals other than laboratory rodents. Methods: We have focused on characterizing the OL in the red fox by performing general and specific histological stainings on serial sections, using both single and double immunohistochemical and lectin-histochemical labeling techniques. Results: As a result, we have been able to determine that the OL of the red fox (Vulpes vulpes) displays an uncommonly high degree of development and complexity. Discussion: This makes this species a novel mammalian model, the study of which could improve our understanding of the noncanonical pathways involved in the processing of chemosensory cues.
Keywords: Canidae; fox; immunohistochemistry; lectins; olfaction; olfactory limbus.
Copyright © 2023 Ortiz-Leal, Torres, Vargas-Barroso, Fidalgo, López-Beceiro, Larriva-Sahd and Sánchez-Quinteiro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alonso J. R., Porteros A., Crespo C., Arevalo R., Briñon J. G., Weruaga E., et al. . (1998). Chemical anatomy of the macaque monkey olfactory bulb: NADPH-diaphorase/nitric oxide synthase activity. J. Comp. Neurol. 402, 419–434. 10.1002/(SICI)1096-9861(19981221)402:3<419::AID-CNE9>3.0.CO;2-C - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

