Involvement of chemokine receptor CXCR3 in the defense mechanism against Neospora caninum infection in C57BL/6 mice
- PMID: 36704563
- PMCID: PMC9873264
- DOI: 10.3389/fmicb.2022.1045106
Involvement of chemokine receptor CXCR3 in the defense mechanism against Neospora caninum infection in C57BL/6 mice
Abstract
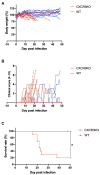
C-X-C motif chemokine receptor 3 (CXCR3) is an important receptor controlling the migration of leukocytes, although there is no report regarding its role in Neospora caninum infection. Herein, we investigated the relevance of CXCR3 in the resistance mechanism to N. caninum infection in mice. Wild-type (WT) C57BL/6 mice and CXCR3-knockout (CXCR3KO) mice were used in all experiments. WT mice displayed a high survival rate (100%), while 80% of CXCR3KO mice succumbed to N. caninum infection within 50 days. Compared with WT mice, CXCR3KO mice exhibited significantly lower body weights and higher clinical scores at the subacute stage of infection. Flow cytometric analysis revealed CXCR3KO mice as having significantly increased proportions and numbers of CD11c-positive cells compared with WT mice at 5 days post infection (dpi). However, levels of interleukin-6 and interferon-γ in serum and ascites were similar in all groups at 5 dpi. Furthermore, no differences in parasite load were detected in brain, spleen, lungs or liver tissue of CXCR3KO and WT mice at 5 and 21 dpi. mRNA analysis of brain tissue collected from infected mice at 30 dpi revealed no changes in expression levels of inflammatory response genes. Nevertheless, the brain tissue of infected CXCR3KO mice displayed significant necrosis and microglial activation compared with that of WT mice at 21 dpi. Interestingly, the brain tissue of CXCR3KO mice displayed significantly lower numbers of FoxP3+ cells compared with the brain tissue of WT mice at 30 dpi. Accordingly, our study suggests that the lack of active regulatory T cells in brain tissue of infected CXCR3KO mice is the main cause of these mice having severe necrosis and lower survival compared with WT mice. Thus, CXCR3+ regulatory T cells may play a crucial role in control of neosporosis.
Keywords: CXCR3; CXCR3KO; IFN-γ; IL-6; Neospora caninum; neosporosis; regulatory T-cells.
Copyright © 2023 Abdelbaky, Mitsuhashi, Watanabe, Ushio, Miyakawa, Furuoka and Nishikawa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Susceptibility of B-cell deficient C57BL/6 (microMT) mice to Neospora caninum infection.Parasite Immunol. 1999 May;21(5):225-36. doi: 10.1046/j.1365-3024.1999.00223.x. Parasite Immunol. 1999. PMID: 10320620
-
ROS-mediated NLRP3 inflammasome activation participates in the response against Neospora caninum infection.Parasit Vectors. 2020 Sep 5;13(1):449. doi: 10.1186/s13071-020-04331-8. Parasit Vectors. 2020. PMID: 32891167 Free PMC article.
-
Predominant role of interferon-γ in the host protective effect of CD8(+) T cells against Neospora caninum infection.Sci Rep. 2015 Oct 9;5:14913. doi: 10.1038/srep14913. Sci Rep. 2015. PMID: 26449650 Free PMC article.
-
Role of the chemokine receptor CCR5-dependent host defense system in Neospora caninum infections.Parasit Vectors. 2015 Jan 6;8:5. doi: 10.1186/s13071-014-0620-5. Parasit Vectors. 2015. PMID: 25558986 Free PMC article.
-
The host-parasite relationship in bovine neosporosis.Vet Immunol Immunopathol. 2005 Oct 18;108(1-2):29-36. doi: 10.1016/j.vetimm.2005.07.004. Vet Immunol Immunopathol. 2005. PMID: 16098610 Review.
Cited by
-
Dietary Fermentation with Lactobacillus sp. and Bacillus sp. Modulates Rumen Transcriptomic and Microbiota Profiles in Bos taurus.Int J Mol Sci. 2025 Jul 16;26(14):6816. doi: 10.3390/ijms26146816. Int J Mol Sci. 2025. PMID: 40725062 Free PMC article.
References
-
- Amin D. N., Rottenberg M., Randrup A., Kristensson K., Masocha W. (2008). CXCL10 and CXCR3 modulate morbidity and brain invasion by parasites and T-cells in an African trypanosomiasis mouse model. BMC Proc. 2:P1. doi: 10.1186/1753-6561-2-S1-P1 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

