Regulation of Photosensitivity by the Hippo Pathway in Lupus Skin
- PMID: 36704840
- PMCID: PMC10313771
- DOI: 10.1002/art.42460
Regulation of Photosensitivity by the Hippo Pathway in Lupus Skin
Abstract
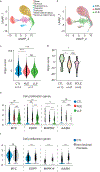
Objective: Photosensitivity is one of the most common manifestations of systemic lupus erythematosus (SLE), yet its pathogenesis is not well understood. The normal-appearing epidermis of patients with SLE exhibits increased ultraviolet B (UVB)-driven cell death that persists in cell culture. Here, we investigated the role of epigenetic modification and Hippo signaling in enhanced UVB-induced apoptosis seen in SLE keratinocytes.
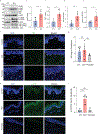
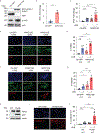
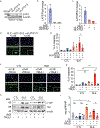
Methods: We analyzed DNA methylation in cultured keratinocytes from SLE patients compared to keratinocytes from healthy controls (n = 6/group). Protein expression was validated in cultured keratinocytes using immunoblotting and immunofluorescence. An immortalized keratinocyte line overexpressing WWC1 was generated via lentiviral vector. WWC1-driven changes were inhibited using a large tumor suppressor kinase 1/2 (LATS1/2) inhibitor (TRULI) and small interfering RNA (siRNA). The interaction between the Yes-associated protein (YAP) and the transcriptional enhancer associate domain (TEAD) was inhibited by overexpression of an N/TERT cell line expressing a tetracycline-inducible green fluorescent protein-tagged protein that inhibits YAP-TEAD binding (TEADi). Apoptosis was assessed using cleaved caspase 3/7 and TUNEL staining.
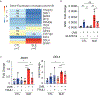
Results: Hippo signaling was the top differentially methylated pathway in SLE versus control keratinocytes. SLE keratinocytes (n = 6) showed significant hypomethylation (Δβ = -0.153) and thus overexpression of the Hippo regulator WWC1 (P = 0.002). WWC1 overexpression increased LATS1/2 kinase activation, leading to YAP cytoplasmic retention and altered proapoptotic transcription in SLE keratinocytes. Accordingly, UVB-mediated apoptosis in keratinocytes could be enhanced by WWC1 overexpression or YAP-TEAD inhibition, mimicking SLE keratinocytes. Importantly, inhibition of LATS1/2 with either the chemical inhibitor TRULI or siRNA effectively eliminated enhanced UVB-apoptosis in SLE keratinocytes.
Conclusion: Our work unravels a novel driver of photosensitivity in SLE: overactive Hippo signaling in SLE keratinocytes restricts YAP transcriptional activity, leading to shifts that promote UVB apoptosis.
© 2023 American College of Rheumatology.
Conflict of interest statement
Figures

Similar articles
-
WWC proteins mediate LATS1/2 activation by Hippo kinases and imply a tumor suppression strategy.Mol Cell. 2022 May 19;82(10):1850-1864.e7. doi: 10.1016/j.molcel.2022.03.027. Epub 2022 Apr 15. Mol Cell. 2022. PMID: 35429439
-
Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation.Nat Cell Biol. 2017 Jul 28;19(8):996-1002. doi: 10.1038/ncb3581. Nat Cell Biol. 2017. PMID: 28752853 Free PMC article.
-
Hippo signaling effectors YAP and TAZ induce Epstein-Barr Virus (EBV) lytic reactivation through TEADs in epithelial cells.PLoS Pathog. 2021 Aug 2;17(8):e1009783. doi: 10.1371/journal.ppat.1009783. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34339458 Free PMC article.
-
Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway.Small GTPases. 2020 Jul;11(4):280-288. doi: 10.1080/21541248.2018.1435986. Epub 2018 Apr 20. Small GTPases. 2020. PMID: 29457552 Free PMC article. Review.
-
Hippo pathway inhibition by blocking the YAP/TAZ-TEAD interface: a patent review.Expert Opin Ther Pat. 2018 Dec;28(12):867-873. doi: 10.1080/13543776.2018.1549226. Epub 2018 Dec 2. Expert Opin Ther Pat. 2018. PMID: 30482112 Review.
Cited by
-
Serum Metabolomics Analysis of Skin-Involved Systemic Lupus Erythematosus: Association of Anti-SSA Antibodies with Photosensitivity.J Inflamm Res. 2023 Aug 30;16:3811-3822. doi: 10.2147/JIR.S426337. eCollection 2023. J Inflamm Res. 2023. PMID: 37667802 Free PMC article.
-
An improved TEAD dominant-negative protein inhibitor to study Hippo YAP1/TAZ-dependent transcription.bioRxiv [Preprint]. 2024 Oct 3:2024.10.03.615022. doi: 10.1101/2024.10.03.615022. bioRxiv. 2024. PMID: 39502361 Free PMC article. Preprint.
-
Dysregulation in keratinocytes drives systemic lupus erythematosus onset.Cell Mol Immunol. 2025 Jan;22(1):83-96. doi: 10.1038/s41423-024-01240-z. Epub 2024 Dec 3. Cell Mol Immunol. 2025. PMID: 39627610
-
Research progress on Hippo signaling pathway effector molecules in rheumatic immune system diseases.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024 Jun 19;53(3):376-381. doi: 10.3724/zdxbyxb-2023-0567. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 38899353 Free PMC article. Review. Chinese, English.
-
HERC6 regulates STING activity in a sex-biased manner through modulation of LATS2/VGLL3 Hippo signaling.iScience. 2024 Jan 23;27(2):108986. doi: 10.1016/j.isci.2024.108986. eCollection 2024 Feb 16. iScience. 2024. PMID: 38327798 Free PMC article.
References
-
- Pirner K, et al. Significance of ultraviolet light in the pathogenesis of systemic lupus erythematosus: case report and discussion of the literature. J Rheumatol. 1992;51(1):20–4. - PubMed
-
- Ansel JC, et al. Effects of UV radiation on autoimmune strains of mice: increased mortality and accelerated autoimmunity in BXSB male mice. J Invest Dermatol. 1985;85(3):181–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 AR077988/AR/NIAMS NIH HHS/United States
- K08 AR060802/AR/NIAMS NIH HHS/United States
- R01 AI130025/AI/NIAID NIH HHS/United States
- R03 AR066337/AR/NIAMS NIH HHS/United States
- T32 GM007863/GM/NIGMS NIH HHS/United States
- K24 AR076975/AR/NIAMS NIH HHS/United States
- T32 AI007413/AI/NIAID NIH HHS/United States
- R01 AR071384/AR/NIAMS NIH HHS/United States
- R01-AR060802/AR/NIAMS NIH HHS/United States
- T32-AR007197/AR/NIAMS NIH HHS/United States
- K08 AR078251/AR/NIAMS NIH HHS/United States
- P30 AR075043/AR/NIAMS NIH HHS/United States
- R03-AR066337/AR/NIAMS NIH HHS/United States
- R01 AR081640/AR/NIAMS NIH HHS/United States
- T32 AR007197/AR/NIAMS NIH HHS/United States
- K24-AR076975/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

