Novel cell culture system for monitoring cells during continuous and variable negative-pressure wound therapy
- PMID: 36704879
- PMCID: PMC9838773
- DOI: 10.1111/srt.13262
Novel cell culture system for monitoring cells during continuous and variable negative-pressure wound therapy
Abstract
Background: Although the clinical efficacy of negative-pressure wound therapy (NPWT) is well known, many of its molecular biological mechanisms remain unresolved, mainly due to the difficulty and paucity of relevant in vitro studies. We attempted to develop an in vitro cell culture system capable of real-time monitoring of cells during NPWT treatment.
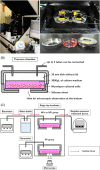
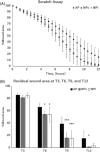
Materials and methods: A novel negative-pressure cell culture system was developed by combining an inverted microscope, a stage-top incubator, a sealed metal chamber for cell culture, and an NPWT treatment device. Human keratinocytes, PSVK-1, were divided into ambient pressure (AP), continuous negative-pressure (NPc), and intermittent negative-pressure (NPi) groups and cultured for 24 h with scratch assay using our real-time monitoring system and device. Pressure inside the device, medium evaporation rate, and the residual wound area were compared across the groups.
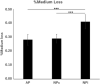
Results: Pressure in the device was maintained at almost the same value as set in all groups. Medium evaporation rate was significantly higher in the NPi group than in the other two groups; however, it had negligible effect on cell culture. Residual wound area after 9 h evaluated by the scratch assay was significantly smaller in the NPc and NPi groups than in the AP group.
Conclusion: We developed a negative-pressure cell culture device that enables negative-pressure cell culture under conditions similar to those used in clinical practice and is able to monitor cells under NPWT. Further experiments using this device would provide high-quality molecular biological evidence for NPWT.
Keywords: epithelial-to-mesenchymal transition; intermittent negative-pressure wound therapy; keratinocyte; microdeformational wound therapy; negative-pressure incubator; topical negative pressure; vacuum-assisted closure; wound healing.
© 2022 The Authors. Skin Research and Technology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Negative-Pressure Wound Therapy: What We Know and What We Need to Know.Adv Exp Med Biol. 2023;1436:131-152. doi: 10.1007/5584_2023_773. Adv Exp Med Biol. 2023. PMID: 36922487
-
In vitro evaluation of a hybrid negative pressure system for wound therapy.Vet Surg. 2024 Aug;53(6):1093-1101. doi: 10.1111/vsu.14101. Epub 2024 May 15. Vet Surg. 2024. PMID: 38747194
-
Negative pressure accelerated monolayer keratinocyte healing involves Cdc42 mediated cell podia formation.J Dermatol Sci. 2013 Jun;70(3):196-203. doi: 10.1016/j.jdermsci.2013.03.007. Epub 2013 Apr 6. J Dermatol Sci. 2013. PMID: 23622765
-
Negative pressure wound therapy for surgical wounds healing by primary closure.Cochrane Database Syst Rev. 2019 Mar 26;3(3):CD009261. doi: 10.1002/14651858.CD009261.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2020 May 1;5:CD009261. doi: 10.1002/14651858.CD009261.pub5. PMID: 30912582 Free PMC article. Updated.
-
EWMA Document: Negative Pressure Wound Therapy.J Wound Care. 2017 Mar 1;26(Sup3):S1-S154. doi: 10.12968/jowc.2017.26.Sup3.S1. J Wound Care. 2017. PMID: 28345371 Review.
Cited by
-
An Automated Microfluidic Platform for In Vitro Raman Analysis of Living Cells.Biosensors (Basel). 2025 Jul 16;15(7):459. doi: 10.3390/bios15070459. Biosensors (Basel). 2025. PMID: 40710109 Free PMC article.
-
Continuous negative-pressure wound therapy improves the survival rate of skin grafts and shortens the time required for skin graft survival.Skin Res Technol. 2024 Jul;30(7):e13865. doi: 10.1111/srt.13865. Skin Res Technol. 2024. PMID: 39031918 Free PMC article.
-
Establishment of the microscope incubation system and its application in evaluating tumor treatment effects through real-time live cellular imaging.Front Bioeng Biotechnol. 2024 Aug 16;12:1447265. doi: 10.3389/fbioe.2024.1447265. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39219621 Free PMC article.
References
-
- Borgquist O, Ingemansson R, Malmsjo M. The effect of intermittent and variable negative pressure wound therapy on wound edge microvascular blood flow. Ostomy Wound Manage. 2010;56:60‐67. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

