Bifidobacterium longum supplementation improves age-related delays in fracture repair
- PMID: 36704918
- PMCID: PMC10086533
- DOI: 10.1111/acel.13786
Bifidobacterium longum supplementation improves age-related delays in fracture repair
Abstract
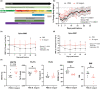
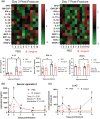
Age-related delays in bone repair remains an important clinical issue that can prolong pain and suffering. It is now well established that inflammation increases with aging and that this exacerbated inflammatory response can influence skeletal regeneration. Recently, simple dietary supplementation with beneficial probiotic bacteria has been shown to influence fracture repair in young mice. However, the contribution of the gut microbiota to age-related impairments in fracture healing remains unknown. Here, we sought to determine whether supplementation with a single beneficial probiotic species, Bifidobacterium longum (B. longum), would promote fracture repair in aged (18-month-old) female mice. We found that B. longum supplementation accelerated bony callus formation which improved mechanical properties of the fractured limb. We attribute these pro-regenerative effects of B. longum to preservation of intestinal barrier, dampened systemic inflammation, and maintenance of the microbiota community structure. Moreover, B. longum attenuated many of the fracture-induced systemic pathologies. Our study provides evidence that targeting the gut microbiota using simple dietary approaches can improve fracture healing outcomes and minimize systemic pathologies in the context of aging.
Keywords: Bifidobacterium longum; aging; femur fracture; gut microbiome; probiotics.
© 2023 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no competing conflicts to disclose.
Figures



Similar articles
-
Bifidobacterium adolescentis supplementation attenuates fracture-induced systemic sequelae.Biomed Pharmacother. 2020 Dec;132:110831. doi: 10.1016/j.biopha.2020.110831. Epub 2020 Oct 3. Biomed Pharmacother. 2020. PMID: 33022534 Free PMC article.
-
Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study.World J Gastroenterol. 2017 Apr 21;23(15):2696-2704. doi: 10.3748/wjg.v23.i15.2696. World J Gastroenterol. 2017. PMID: 28487606 Free PMC article. Clinical Trial.
-
Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI promotes neuronal rejuvenation in aged mice.Biochem Biophys Res Commun. 2022 May 7;603:41-48. doi: 10.1016/j.bbrc.2022.03.024. Epub 2022 Mar 5. Biochem Biophys Res Commun. 2022. PMID: 35278878
-
Efficacy of Bifidobacterium longum alone or in multi-strain probiotic formulations during early life and beyond.Gut Microbes. 2023 Jan-Dec;15(1):2186098. doi: 10.1080/19490976.2023.2186098. Gut Microbes. 2023. PMID: 36896934 Free PMC article. Review.
-
Bosom Buddies: The Symbiotic Relationship Between Infants and Bifidobacterium longum ssp. longum and ssp. infantis. Genetic and Probiotic Features.Annu Rev Food Sci Technol. 2016;7:1-21. doi: 10.1146/annurev-food-041715-033151. Annu Rev Food Sci Technol. 2016. PMID: 26934170 Review.
Cited by
-
Microbiota-bone axis in ageing-related bone diseases.Front Endocrinol (Lausanne). 2024 Jul 15;15:1414350. doi: 10.3389/fendo.2024.1414350. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39076510 Free PMC article. Review.
-
Systemic inflammatory and gut microbiota responses to fracture in young and middle-aged mice.Geroscience. 2023 Dec;45(6):3115-3129. doi: 10.1007/s11357-023-00963-7. Epub 2023 Oct 12. Geroscience. 2023. PMID: 37821753 Free PMC article.
-
Probiotics: Can it modulate fracture healing?PLoS One. 2023 Aug 31;18(8):e0290738. doi: 10.1371/journal.pone.0290738. eCollection 2023. PLoS One. 2023. PMID: 37651346 Free PMC article.
-
IL-17RA Signaling in Prx1+ Mesenchymal Cells Influences Fracture Healing in Mice.Int J Mol Sci. 2024 Mar 28;25(7):3751. doi: 10.3390/ijms25073751. Int J Mol Sci. 2024. PMID: 38612562 Free PMC article.
-
Achieving healthy aging through gut microbiota-directed dietary intervention: Focusing on microbial biomarkers and host mechanisms.J Adv Res. 2025 Feb;68:179-200. doi: 10.1016/j.jare.2024.03.005. Epub 2024 Mar 9. J Adv Res. 2025. PMID: 38462039 Free PMC article. Review.
References
-
- Ahmadi, S. , Razazan, A. , Nagpal, R. , Jain, S. , Wang, B. , Mishra, S. P. , Wang, S. , Justice, J. , Ding, J. , McClain, D. , Kritchevsky, S. B. , Kitzman, D. , & Yadav, H. (2020). Metformin reduces aging‐related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin Axis. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(7), e9–e21. 10.1093/gerona/glaa056 - DOI - PMC - PubMed
-
- Ajmo, C. T., Jr. , Collier, L. A. , Leonardo, C. C. , Hall, A. A. , Green, S. M. , Womble, T. A. , Cuevas, J. , Willing, A. E. , & Pennypacker, K. R. (2009). Blockade of adrenoreceptors inhibits the splenic response to stroke. Experimental Neurology, 218(1), 47–55. 10.1016/j.expneurol.2009.03.044 - DOI - PMC - PubMed
-
- Al‐Sadi, R. , Ye, D. , Boivin, M. , Guo, S. , Hashimi, M. , Ereifej, L. , & Ma, T. Y. (2014). Interleukin‐6 modulation of intestinal epithelial tight junction permeability is mediated by JNK pathway activation of claudin‐2 gene. PLoS One, 9(3), e85345. 10.1371/journal.pone.0085345 - DOI - PMC - PubMed
-
- Bellissimo, M. P. , Ziegler, T. R. , Jones, D. P. , Liu, K. H. , Fernandes, J. , Roberts, J. L. , Weitzmann, M. N. , Pacifici, R. , & Alvarez, J. A. (2021). Plasma high‐resolution metabolomics identifies linoleic acid and linked metabolic pathways associated with bone mineral density. Clinical Nutrition, 40(2), 467–475. 10.1016/j.clnu.2020.05.041 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

