Blockage of autophagosome-lysosome fusion through SNAP29 O-GlcNAcylation promotes apoptosis via ROS production
- PMID: 36704963
- PMCID: PMC10283446
- DOI: 10.1080/15548627.2023.2170962
Blockage of autophagosome-lysosome fusion through SNAP29 O-GlcNAcylation promotes apoptosis via ROS production
Abstract
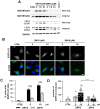
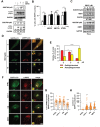
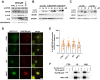
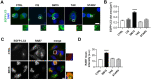
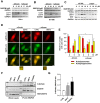
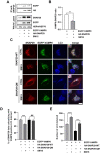
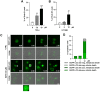
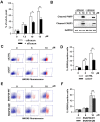
Macroautophagy/autophagy has been shown to exert a dual role in cancer i.e., promoting cell survival or cell death depending on the cellular context and the cancer stage. Therefore, development of potent autophagy modulators, with a clear mechanistic understanding of their target action, has paramount importance in both mechanistic and clinical studies. In the process of exploring the mechanism of action of a previously identified cytotoxic small molecule (SM15) designed to target microtubules and the interaction domain of microtubules and the kinetochore component NDC80/HEC1, we discovered that the molecule acts as a potent autophagy inhibitor. By using several biochemical and cell biology assays we demonstrated that SM15 blocks basal autophagic flux by inhibiting the fusion of correctly formed autophagosomes with lysosomes. SM15-induced autophagic flux blockage promoted apoptosis-mediated cell death associated with ROS production. Interestingly, autophagic flux blockage, apoptosis induction and ROS production were rescued by genetic or pharmacological inhibition of OGT (O-linked N-acetylglucosamine (GlcNAc) transferase) or by expressing an O-GlcNAcylation-defective mutant of the SNARE fusion complex component SNAP29, pointing to SNAP29 as the molecular target of SM15 in autophagy. Accordingly, SM15 was found to enhance SNAP29 O-GlcNAcylation and, thereby, inhibit the formation of the SNARE fusion complex. In conclusion, these findings identify a new pathway in autophagy connecting O-GlcNAcylated SNAP29 to autophagic flux blockage and autophagosome accumulation, that, in turn, drives ROS production and apoptotic cell death. Consequently, modulation of SNAP29 activity may represent a new opportunity for therapeutic intervention in cancer and other autophagy-associated diseases.
Keywords: Anticancer therapy; O-GlcNAcylation; SNAP29; apoptosis; autophagic flux; autophagy; kinetochore; reactive oxygen species.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Human YKT6 forms priming complex with STX17 and SNAP29 to facilitate autophagosome-lysosome fusion.Cell Rep. 2024 Feb 27;43(2):113760. doi: 10.1016/j.celrep.2024.113760. Epub 2024 Feb 9. Cell Rep. 2024. PMID: 38340317
-
Increased O-GlcNAcylation of SNAP29 Drives Arsenic-Induced Autophagic Dysfunction.Mol Cell Biol. 2018 May 15;38(11):e00595-17. doi: 10.1128/MCB.00595-17. Print 2018 Jun 1. Mol Cell Biol. 2018. PMID: 29507186 Free PMC article.
-
New insights regarding SNARE proteins in autophagosome-lysosome fusion.Autophagy. 2021 Oct;17(10):2680-2688. doi: 10.1080/15548627.2020.1823124. Epub 2020 Sep 24. Autophagy. 2021. PMID: 32924745 Free PMC article. Review.
-
RUNDC1 negatively mediates the fusion of autophagosomes with lysosomes via regulating SNARE complex assembly.Autophagy. 2024 Feb;20(2):454-456. doi: 10.1080/15548627.2023.2274210. Epub 2024 Jan 25. Autophagy. 2024. PMID: 37876308 Free PMC article.
-
Evolutionarily conserved role and physiological relevance of a STX17/Syx17 (syntaxin 17)-containing SNARE complex in autophagosome fusion with endosomes and lysosomes.Autophagy. 2013 Oct;9(10):1642-6. doi: 10.4161/auto.25684. Epub 2013 Jul 22. Autophagy. 2013. PMID: 24113031 Review.
Cited by
-
Metabolomics combined with network pharmacology reveals a role for astragaloside IV in inhibiting enterovirus 71 replication via PI3K-AKT signaling.J Transl Med. 2024 Jun 10;22(1):555. doi: 10.1186/s12967-024-05355-9. J Transl Med. 2024. PMID: 38858642 Free PMC article.
-
Biomarkers in Hereditary Spastic Paraplegias.Int J Mol Sci. 2025 Feb 24;26(5):1950. doi: 10.3390/ijms26051950. Int J Mol Sci. 2025. PMID: 40076577 Free PMC article. Review.
-
DHCR24 in Tumor Diagnosis and Treatment: A Comprehensive Review.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241259780. doi: 10.1177/15330338241259780. Technol Cancer Res Treat. 2024. PMID: 38847653 Free PMC article. Review.
-
Role of O-linked N-acetylglucosamine protein modification in oxidative stress-induced autophagy: a novel target for bone remodeling.Cell Commun Signal. 2024 Jul 10;22(1):358. doi: 10.1186/s12964-024-01734-3. Cell Commun Signal. 2024. PMID: 38987770 Free PMC article. Review.
-
Molecular Mechanism of Autophagosome-Lysosome Fusion in Mammalian Cells.Cells. 2024 Mar 13;13(6):500. doi: 10.3390/cells13060500. Cells. 2024. PMID: 38534345 Free PMC article. Review.
References
-
- Dikic I, Elazar Z.. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19(6):349–364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
