Palladium-Catalyzed Carbonylations: Application in Complex Natural Product Total Synthesis and Recent Developments
- PMID: 36705327
- PMCID: PMC10127288
- DOI: 10.1021/acs.joc.2c02746
Palladium-Catalyzed Carbonylations: Application in Complex Natural Product Total Synthesis and Recent Developments
Abstract
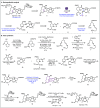
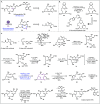
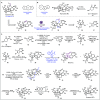
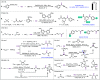
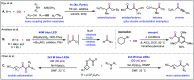
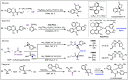
Carbon monoxide is a cheap and abundant C1 building block that can be readily incorporated into organic molecules to rapidly build structural complexity. In this Perspective, we outline several recent (since 2015) examples of palladium-catalyzed carbonylations in streamlining complex natural product total synthesis and highlight the strategic importance of these carbonylation reactions in the corresponding synthesis. The selected examples include spinosyn A, callyspongiolide, perseanol, schizozygane alkaloids, cephanolides, and bisdehydroneostemoninine and related stemona alkaloids. We also provide our perspective about the recent advancements and future developments of palladium-catalyzed carbonylations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




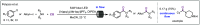


Similar articles
-
Natural Product Synthesis via Palladium-Catalyzed Carbonylation.J Org Chem. 2017 Mar 3;82(5):2319-2328. doi: 10.1021/acs.joc.7b00009. Epub 2017 Feb 13. J Org Chem. 2017. PMID: 28170262
-
Transition-metal-catalyzed carbonylation reactions of olefins and alkynes: a personal account.Acc Chem Res. 2014 Apr 15;47(4):1041-53. doi: 10.1021/ar400222k. Epub 2014 Feb 24. Acc Chem Res. 2014. PMID: 24564478
-
Palladium-catalyzed carbonylations of aryl bromides using paraformaldehyde: synthesis of aldehydes and esters.Angew Chem Int Ed Engl. 2014 Sep 15;53(38):10090-4. doi: 10.1002/anie.201404833. Epub 2014 Jul 28. Angew Chem Int Ed Engl. 2014. PMID: 25070708
-
Palladium-catalyzed oxidative carbonylation reactions.ChemSusChem. 2013 Feb;6(2):229-41. doi: 10.1002/cssc.201200683. Epub 2013 Jan 10. ChemSusChem. 2013. PMID: 23307763 Review.
-
Supported Palladium Catalyzed Carbonylative Coupling Reactions using Carbon Monoxide as C1 Source.Chem Rec. 2022 Jan;22(1):e202100157. doi: 10.1002/tcr.202100157. Epub 2021 Aug 21. Chem Rec. 2022. PMID: 34418288 Review.
Cited by
-
Heck Macrocyclization in Forging Non-Natural Large Rings including Macrocyclic Drugs.Int J Mol Sci. 2023 May 4;24(9):8252. doi: 10.3390/ijms24098252. Int J Mol Sci. 2023. PMID: 37175956 Free PMC article. Review.
-
Competing Mechanisms in Palladium-Catalyzed Alkoxycarbonylation of Styrene.ACS Catal. 2024 Apr 1;14(8):5710-5719. doi: 10.1021/acscatal.4c00966. eCollection 2024 Apr 19. ACS Catal. 2024. PMID: 38660606 Free PMC article.
-
Co2(CO)8 as a CO-source for Pd-catalyzed Carbonylations: An Update.Curr Org Synth. 2025;22(4):421-438. doi: 10.2174/0115701794302069240624045929. Curr Org Synth. 2025. PMID: 40420782
-
A Tale of Two Cities in Fluorescent Sensing of Carbon Monoxide: Probes That Detect CO and Those That Detect Only Chemically Reactive CO Donors (CORMs), but Not CO.J Org Chem. 2024 Dec 20;89(24):17891-17909. doi: 10.1021/acs.joc.4c02301. Epub 2024 Nov 14. J Org Chem. 2024. PMID: 39540647 Free PMC article. Review.
-
Fe3O4@SiO2-DHB/DI(S-NH)-Pd(0) nanocomposite: a novel, efficient, and reusable heterogeneous catalyst for carbonylative preparation of N-aryl amides.BMC Chem. 2025 Mar 15;19(1):71. doi: 10.1186/s13065-025-01440-2. BMC Chem. 2025. PMID: 40089717 Free PMC article.
References
-
- Peng J.-B.; Geng H.-Q.; Wu X.-F. The Chemistry of CO: Carbonylation. Chem. 2019, 5, 526–552. 10.1016/j.chempr.2018.11.006. - DOI
- Gabriele B.Carbon Monoxide in Organic Synthesis: Carbonylation Chemistry; Wiley-VCH: Weinheim, 2021.
- Zhao S.; Mankad N. P. Metal-catalysed radical carbonylation reactions. Catal. Sci. Technol. 2019, 9, 3603–3613. 10.1039/C9CY00938H. - DOI
- Fujimori S.; Inoue S. Carbon Monoxide in Main-Group Chemistry. J. Am. Chem. Soc. 2022, 144, 2034–2050. 10.1021/jacs.1c13152. - DOI - PubMed
-
- Schoenberg A.; Heck R. F. Palladium-catalyzed amidation of aryl, heterocyclic, and vinylic Halides. J. Org. Chem. 1974, 39, 3327–3331. 10.1021/jo00937a004. - DOI
- Schoenberg A.; Bartoletti I.; Heck R. F. Palladium-catalyzed carboalkoxylation of aryl, benzyl, and vinylic halides. J. Org. Chem. 1974, 39, 3318–3326. 10.1021/jo00937a003. - DOI
-
- Wu X.-F.; Neumann H.; Beller M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. 10.1021/cr300100s. - DOI - PubMed
- Barnard C. F. J. Palladium-Catalyzed Carbonylation - A Reaction Come of Age. Organometallics 2008, 27, 5402–5422. 10.1021/om800549q. - DOI
- Fang W.; Zhu H.; Deng Q.; Liu S.; Liu X.; Shen Y.; Tu T. Design and Development of Ligands for Palladium-Catalyzed Carbonylation Reactions. Synthesis 2014, 46, 1689–1708. 10.1055/s-0033-1338635. - DOI
-
- Semmelhack M. F.; Bodurow C. Intramolecular alkoxypalladation/carbonylation of alkenes. J. Am. Chem. Soc. 1984, 106, 1496–1498. 10.1021/ja00317a059. - DOI
- Semmelhack M. F.; Kim C.; Zhang N.; Bodurow C.; Sanner M.; Dobler W.; Meier M. Intramolecular alkoxy-carbonylation of hydroxy alkenes promoted by Pd(II). Pure Appl. Chem. 1990, 62, 2035–2040. 10.1351/pac199062102035. - DOI
-
- Bai Y.; Davis D. C.; Dai M. Natural Product Synthesis via Palladium-Catalyzed Carbonylation. J. Org. Chem. 2017, 82, 2319–2328. 10.1021/acs.joc.7b00009. - DOI - PubMed
- Ma K.; Martin B. S.; Yin X.; Dai M. Natural product syntheses via carbonylative cyclizations. Nat. Prod. Rep. 2019, 36, 174–219. 10.1039/C8NP00033F. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

