Growth differences by school-age and adolescence according to in utero and peripartum antiretroviral therapy exposure among Ugandan children
- PMID: 36705393
- PMCID: PMC9875969
- DOI: 10.1097/MD.0000000000032677
Growth differences by school-age and adolescence according to in utero and peripartum antiretroviral therapy exposure among Ugandan children
Abstract
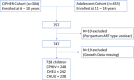
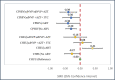
In utero/peripartum antiretroviral (IPA) drug exposure in human immunodeficiency virus (HIV)-exposed children has established benefit for prevention of HIV mother-to-child-transmission but its association with height-for-age by adolescence is unknown. Hence we quantify IPA-associated growth differences at 6 to 18 years old among children with perinatally acquired HIV (CPHIV) infection and children HIV exposed but uninfected (CHEU) relative to children HIV unexposed and uninfected (CHUU). Cohort study. Kampala, Uganda. Two hundred thirty eight community controls and 490 children of women living with HIV born between 2000 and 2011 in a community were enrolled at 6 to 18 years of age and followed every 6 months for 1 year. Height-for-age determined at enrollment, 6 and 12 months after enrollment using the World Health Organization reference. IPA exposure was retrospectively determined from medical records and categorized as: no IPA, single-dose nevirapine with/without zidovudine (sdNVP ± AZT), sdNVP + AZT + lamivudine, or combination antiretroviral therapy (cART). Mean differences (β) with 95% confidence intervals (CIs) in height-for-age over 12 months were evaluated according to IPA exposure for CPHIV and CHEU and relative to CHUU using longitudinal linear mixed effects models adjusted for caregiver factors (sex, age, education, functioning in caregiving role, and lifetime adversity) in Statistical Analysis Software (v.9.4). Regardless of IPA type, CPHIV grew worse than CHUU by school-age/adolescence (β = -0.30, 95% CI: -0.48, -0.11). Relative to CHUU height-for-age was similar for CHEU exposed to sdNVP ± AZT (β = -0.16, 95% CI: -0.46, 0.14) and for CHEU exposed to sdNVP + AZT + lamivudine (β = 0.08, 95% CI: -0.20, 0.35). However, CHEU without any IPA exposure had lower height-for-age (β = -0.27, 95% CI: -0.52, -0.00) whereas CHEU with cART exposure had greater height-for-age (β = 0.41, 95% CI: 0.10, 0.71) in comparison with CHUU by 6 to 18 years old. Our findings suggest that CHEU may achieve height-for-age parity with CHUU by school-age and adolescent years- especially if provided benefit of effective cART in the peripartum period. However, CPHIV regardless of IPA exposure type and CHEU without IPA exposure remain at a disadvantage and will benefit from intervention to support their growth.
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
In utero/peripartum antiretroviral therapy exposure and mental health outcomes at 8-18 years old: A longitudinal comparative study of children with perinatally acquired HIV, children perinatally HIV exposed but uninfected, and children unexposed uninfected from Uganda.Res Nurs Health. 2024 Apr;47(2):195-207. doi: 10.1002/nur.22359. Epub 2023 Nov 30. Res Nurs Health. 2024. PMID: 38031814
-
Developmental Disorder Probability Scores at 6-18 Years Old in Relation to In-Utero/Peripartum Antiretroviral Drug Exposure among Ugandan Children.Int J Environ Res Public Health. 2022 Mar 21;19(6):3725. doi: 10.3390/ijerph19063725. Int J Environ Res Public Health. 2022. PMID: 35329408 Free PMC article.
-
In utero and peripartum antiretroviral exposure as predictor of cognition in 6- to 10-year-old HIV-exposed Ugandan children - a prospective cohort study.HIV Med. 2021 Aug;22(7):592-604. doi: 10.1111/hiv.13094. Epub 2021 Apr 16. HIV Med. 2021. PMID: 33860626 Free PMC article.
-
Neurological development of children who are HIV-exposed and uninfected.Dev Med Child Neurol. 2021 Oct;63(10):1161-1170. doi: 10.1111/dmcn.14921. Epub 2021 May 14. Dev Med Child Neurol. 2021. PMID: 33987826 Review.
-
Antiretroviral therapy during pregnancy and early neonatal life: consequences for HIV-exposed, uninfected children.Braz J Infect Dis. 2004 Apr;8(2):140-50. doi: 10.1590/s1413-86702004000200004. Epub 2004 Sep 8. Braz J Infect Dis. 2004. PMID: 15361992 Review.
References
-
- Darby A, Jones SH. World Health Organization Guidelines (Option A, B, and B+) for Antiretroviral Drugs to Treat Pregnant Women and Prevent HIV Infection in Infants. Embryo Project Encyclopedia. 2021. Available at: http://embryo.asu.edu/handle/10776/13231.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

