What Drives Chorismate Mutase to Top Performance? Insights from a Combined In Silico and In Vitro Study
- PMID: 36705397
- PMCID: PMC9910054
- DOI: 10.1021/acs.biochem.2c00635
What Drives Chorismate Mutase to Top Performance? Insights from a Combined In Silico and In Vitro Study
Abstract
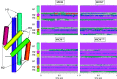
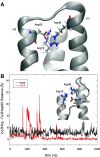
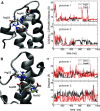
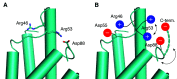
Unlike typical chorismate mutases, the enzyme from Mycobacterium tuberculosis (MtCM) has only low activity on its own. Remarkably, its catalytic efficiency kcat/Km can be boosted more than 100-fold by complex formation with a partner enzyme. Recently, an autonomously fully active MtCM variant was generated using directed evolution, and its structure was solved by X-ray crystallography. However, key residues were involved in crystal contacts, challenging the functional interpretation of the structural changes. Here, we address these challenges by microsecond molecular dynamics simulations, followed up by additional kinetic and structural analyses of selected sets of specifically engineered enzyme variants. A comparison of wild-type MtCM with naturally and artificially activated MtCMs revealed the overall dynamic profiles of these enzymes as well as key interactions between the C-terminus and the active site loop. In the artificially evolved variant of this model enzyme, this loop is preorganized and stabilized by Pro52 and Asp55, two highly conserved residues in typical, highly active chorismate mutases. Asp55 stretches across the active site and helps to appropriately position active site residues Arg18 and Arg46 for catalysis. The role of Asp55 can be taken over by another acidic residue, if introduced at position 88 close to the C-terminus of MtCM, as suggested by molecular dynamics simulations and confirmed by kinetic investigations of engineered variants.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

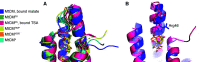

Similar articles
-
Evolving the naturally compromised chorismate mutase from Mycobacterium tuberculosis to top performance.J Biol Chem. 2020 Dec 18;295(51):17514-17534. doi: 10.1074/jbc.RA120.014924. J Biol Chem. 2020. PMID: 33453995 Free PMC article.
-
Structure and function of a complex between chorismate mutase and DAHP synthase: efficiency boost for the junior partner.EMBO J. 2009 Jul 22;28(14):2128-42. doi: 10.1038/emboj.2009.165. Epub 2009 Jun 25. EMBO J. 2009. PMID: 19556970 Free PMC article.
-
A novel noncovalent complex of chorismate mutase and DAHP synthase from Mycobacterium tuberculosis: protein purification, crystallization and X-ray diffraction analysis.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009 Oct 1;65(Pt 10):1048-52. doi: 10.1107/S1744309109035878. Epub 2009 Sep 25. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009. PMID: 19851019 Free PMC article.
-
Pericyclic reactions catalyzed by chorismate-utilizing enzymes.Biochemistry. 2011 Sep 6;50(35):7476-83. doi: 10.1021/bi2009739. Epub 2011 Aug 12. Biochemistry. 2011. PMID: 21823653 Free PMC article. Review.
-
Mycobacterium tuberculosis chorismate mutase: A potential target for TB.Bioorg Med Chem. 2017 Mar 15;25(6):1725-1736. doi: 10.1016/j.bmc.2017.02.001. Epub 2017 Feb 4. Bioorg Med Chem. 2017. PMID: 28202315 Review.
Cited by
-
Aromatic Amino Acids: Exploring Microalgae as a Potential Biofactory.BioTech (Basel). 2025 Jan 29;14(1):6. doi: 10.3390/biotech14010006. BioTech (Basel). 2025. PMID: 39982273 Free PMC article. Review.
References
-
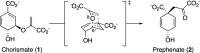
- Sogo S. G.; Widlanski T. S.; Hoare J. H.; Grimshaw C. E.; Berchtold G. A.; Knowles J. R. Stereochemistry of the rearrangement of chorismate to prephenate: Chorismate mutase involves a chair transition state. J. Am. Chem. Soc. 1984, 106, 2701–2703. 10.1021/ja00321a039. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

