Berberine promotes immunological outcomes and decreases neuroinflammation in the experimental model of multiple sclerosis through the expansion of Treg and Th2 cells
- PMID: 36705421
- PMCID: PMC9837936
- DOI: 10.1002/iid3.766
Berberine promotes immunological outcomes and decreases neuroinflammation in the experimental model of multiple sclerosis through the expansion of Treg and Th2 cells
Abstract
Introduction: Among the most frequent demyelinating autoimmune disorders of the central nervous system (CNS) is multiple sclerosis. Experimental autoimmune encephalomyelitis (EAE) is used as an animal model of multiple sclerosis. Berberine is an alkaloid found in some medicinal plants with anti-inflammatory effects.
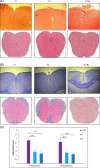
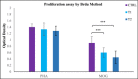
Methods: C57BL/6 female mice were used and divided into three groups: (1) The control group received PBS, (2) the low-dose treatment group received 10 mg/kg of berberine, and (3) The high-dose treatment group received 30 mg/kg of berberine. Myelin Oligodendrocyte Glycoprotein and complete Freund's adjuvant were subcutaneously administered to induce EAE. Mice were given intraperitoneal injections of pertussis toxin on the day of immunization and 2 days later. Histological studies showed low lymphocyte infiltration and demyelination of CNS in the treated groups.
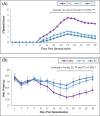
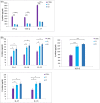
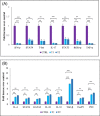
Results: The clinical scores of the treatment group with low-dose berberine (T1: 2 ± 0.13) and high-dose berberine (T2: 1.5 ± 0.14) were significantly (p < .001) lower than the control group (CTRL: 4.5 ± 0.13). Treatment groups decreased pro-inflammatory cytokines (IFN-γ, TNF-α, interleukin [IL]-17) (p < .001) as well as increased anti-inflammatory cytokine expression (IL-4, IL-10, IL-27, IL-33, IL-35, TGF-β) (p < .01) when compared to the CTRL group. Treatment groups with berberine reduced expression of the Th1 and Th17 cytokines and transcription factors (p < .001) and increased expression of transcription factors and Th2 and Treg cytokines (p < .01) in contrast to CTRL group.
Conclusion: Berberine appears to have a protective effect on disease development and alleviating disease status in EAE, which appears to be due to the cell expansion and function of Treg and Th2 cells in addition to berberine's anti-inflammatory properties.
Keywords: Berberine; experimental autoimmune encephalomyelitis; multiple sclerosis; myelin oligodendrocyte glycoprotein.
© 2023 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Berberine: A natural modulator of immune cells in multiple sclerosis.Immun Inflamm Dis. 2024 Mar;12(3):e1213. doi: 10.1002/iid3.1213. Immun Inflamm Dis. 2024. PMID: 38477663 Free PMC article. Review.
-
Adipose-derived mesenchymal stem cells ameliorates experimental autoimmune encephalomyelitis via modulation of Th1/Th17 and expansion of Th2/Treg responses.Cell Biol Int. 2024 Aug;48(8):1124-1137. doi: 10.1002/cbin.12171. Epub 2024 May 14. Cell Biol Int. 2024. PMID: 38741520
-
Daphnetin alleviates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells and upregulating Th2 and regulatory T cells.Acta Neurobiol Exp (Wars). 2022;82(3):273-283. doi: 10.55782/ane-2022-026. Acta Neurobiol Exp (Wars). 2022. PMID: 36214710
-
Pregnancy level of estrogen attenuates experimental autoimmune encephalomyelitis in both ovariectomized and pregnant C57BL/6 mice through expansion of Treg and Th2 cells.J Neuroimmunol. 2014 Dec 15;277(1-2):85-95. doi: 10.1016/j.jneuroim.2014.10.004. Epub 2014 Oct 18. J Neuroimmunol. 2014. PMID: 25457839
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
Cited by
-
Regulatory T Cells in Multiple Sclerosis Diagnostics-What Do We Know So Far?J Pers Med. 2023 Dec 25;14(1):29. doi: 10.3390/jpm14010029. J Pers Med. 2023. PMID: 38248730 Free PMC article. Review.
-
Berberine: A natural modulator of immune cells in multiple sclerosis.Immun Inflamm Dis. 2024 Mar;12(3):e1213. doi: 10.1002/iid3.1213. Immun Inflamm Dis. 2024. PMID: 38477663 Free PMC article. Review.
-
Curcumin's spice-infused therapeutic promise: disease severity alleviation in a mouse model of multiple sclerosis via modulation of immune responses.Mol Biol Rep. 2023 Nov;50(11):8843-8853. doi: 10.1007/s11033-023-08781-y. Epub 2023 Sep 3. Mol Biol Rep. 2023. PMID: 37660318
-
NLRP3 inflammasome in neuroinflammation and central nervous system diseases.Cell Mol Immunol. 2025 Apr;22(4):341-355. doi: 10.1038/s41423-025-01275-w. Epub 2025 Mar 13. Cell Mol Immunol. 2025. PMID: 40075143 Free PMC article. Review.
-
The IL-12 family cytokines in neurodegenerative diseases: dual roles in neurotoxicity and neuroprotection.Inflammopharmacology. 2025 Aug 13. doi: 10.1007/s10787-025-01901-z. Online ahead of print. Inflammopharmacology. 2025. PMID: 40801981 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

