Adjuvant Temozolomide Chemotherapy With or Without Interferon Alfa Among Patients With Newly Diagnosed High-grade Gliomas: A Randomized Clinical Trial
- PMID: 36705923
- PMCID: PMC11839150
- DOI: 10.1001/jamanetworkopen.2022.53285
Adjuvant Temozolomide Chemotherapy With or Without Interferon Alfa Among Patients With Newly Diagnosed High-grade Gliomas: A Randomized Clinical Trial
Abstract
Importance: High-grade gliomas (HGGs) constitute the most common and aggressive primary brain tumor, with 5-year survival rates of 30.9% for grade 3 gliomas and 6.6% for grade 4 gliomas. The add-on efficacy of interferon alfa is unclear for the treatment of HGG.
Objectives: To compare the therapeutic efficacy and toxic effects of the combination of temozolomide and interferon alfa and temozolomide alone in patients with newly diagnosed HGG.
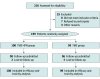
Design, setting, and participants: This multicenter, randomized, phase 3 clinical trial enrolled 199 patients with newly diagnosed HGG from May 1, 2012, to March 30, 2016, at 15 Chinese medical centers. Follow-up was completed July 31, 2021, and data were analyzed from September 13 to November 24, 2021. Eligible patients were aged 18 to 75 years with newly diagnosed and histologically confirmed HGG and had received no prior chemotherapy, radiotherapy, or immunotherapy for their HGG.
Interventions: All patients received standard radiotherapy concurrent with temozolomide. After a 4-week break, patients in the temozolomide with interferon alfa group received standard temozolomide combined with interferon alfa every 28 days. Patients in the temozolomide group received standard temozolomide.
Main outcomes and measures: The primary end point was 2-year overall survival (OS). Secondary end points were 2-year progression-free survival (PFS) and treatment tolerability.
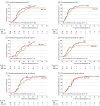
Results: A total of 199 patients with HGG were enrolled, with a median follow-up time of 66.0 (95% CI, 59.1-72.9) months. Seventy-nine patients (39.7%) were women and 120 (60.3%) were men, with ages ranging from 18 to 75 years and a median age of 46.9 (95% CI, 45.3-48.7) years. The median OS of patients in the temozolomide plus interferon alfa group (26.7 [95% CI, 21.6-31.7] months) was significantly longer than that in the standard group (18.8 [95% CI, 16.9-20.7] months; hazard ratio [HR], 0.64 [95% CI, 0.47-0.88]; P = .005). Temozolomide plus interferon alfa also significantly improved median OS in patients with O6-methylguanine-DNA methyltransferase (MGMT) unmethylation (24.7 [95% CI, 20.5-28.8] months) compared with temozolomide (17.4 [95% CI, 14.1-20.7] months; HR, 0.57 [95% CI, 0.37-0.87]; P = .008). Seizure and influenzalike symptoms were more common in the temozolomide plus interferon alfa group, with 2 of 100 (2.0%) and 5 of 100 (5.0%) patients with grades 1 and 2 toxic effects, respectively (P = .02). Finally, results suggested that methylation level at the IFNAR1/2 promoter was a marker of sensitivity to temozolomide plus interferon alfa.
Conclusions and relevance: Compared with the standard regimen, temozolomide plus interferon alfa treatment could prolong the survival time of patients with HGG, especially the MGMT promoter unmethylation variant, and the toxic effects remained tolerable.
Trial registration: ClinicalTrials.gov Identifier: NCT01765088.
Conflict of interest statement
Figures

Similar articles
-
The outcomes of concomitant radiation plus temozolomide followed by adjuvant temozolomide for newly diagnosed high grade gliomas: the preliminary results of single center prospective study.J Egypt Natl Canc Inst. 2009 Jun;21(2):107-19. J Egypt Natl Canc Inst. 2009. PMID: 21057562 Clinical Trial.
-
Phase II trial of 7 days on/7 days off temozolmide for recurrent high-grade glioma.Neuro Oncol. 2014 Sep;16(9):1255-62. doi: 10.1093/neuonc/nou044. Epub 2014 Mar 26. Neuro Oncol. 2014. PMID: 24670608 Free PMC article. Clinical Trial.
-
Association of MGMT Promoter Methylation Status With Survival Outcomes in Patients With High-Risk Glioma Treated With Radiotherapy and Temozolomide: An Analysis From the NRG Oncology/RTOG 0424 Trial.JAMA Oncol. 2018 Oct 1;4(10):1405-1409. doi: 10.1001/jamaoncol.2018.1977. JAMA Oncol. 2018. PMID: 29955793 Free PMC article. Clinical Trial.
-
Anti-angiogenic therapy for high-grade glioma.Cochrane Database Syst Rev. 2018 Nov 22;11(11):CD008218. doi: 10.1002/14651858.CD008218.pub4. Cochrane Database Syst Rev. 2018. PMID: 30480778 Free PMC article.
-
Narrative review of palliative hypofractionated radiotherapy for high grade glioma.Ann Palliat Med. 2021 Jan;10(1):846-862. doi: 10.21037/apm-20-1246. Epub 2020 Sep 22. Ann Palliat Med. 2021. PMID: 33040565 Review.
Cited by
-
Single-cell transcriptomics reveals IRF7 regulation of the tumor microenvironment in isocitrate dehydrogenase wild-type glioma.MedComm (2020). 2024 Nov 3;5(11):e754. doi: 10.1002/mco2.754. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492838 Free PMC article.
-
The role of radiotherapy in immunotherapy strategies in the central nervous system.Neuro Oncol. 2024 Mar 4;26(12 Suppl 2):S66-S75. doi: 10.1093/neuonc/noad184. Neuro Oncol. 2024. PMID: 38437664 Free PMC article. Review.
-
Therapeutic Targets in Glioblastoma: Molecular Pathways, Emerging Strategies, and Future Directions.Cells. 2025 Mar 26;14(7):494. doi: 10.3390/cells14070494. Cells. 2025. PMID: 40214448 Free PMC article. Review.
-
Revolutionizing Brain Tumor Care: Emerging Technologies and Strategies.Biomedicines. 2024 Jun 20;12(6):1376. doi: 10.3390/biomedicines12061376. Biomedicines. 2024. PMID: 38927583 Free PMC article. Review.
-
Targeting Innate Immunity in Glioma Therapy.Int J Mol Sci. 2024 Jan 12;25(2):947. doi: 10.3390/ijms25020947. Int J Mol Sci. 2024. PMID: 38256021 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. doi:10.1056/NEJMoa043330 - DOI - PubMed
-
- Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. doi:10.1016/S1470-2045(09)70025-7 - DOI - PubMed
-
- Yang QY, Shen D, Sai K, et al. . Survival of newly diagnosed malignant glioma patients on combined modality therapy. Article in Chinese. Zhonghua Yi Xue Za Zhi. 2013;93(1):8-10. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

