Carotid dysfunction in senescent female mice is mediated by increased α1A-adrenoceptor activity and COX-derived vasoconstrictor prostanoids
- PMID: 36705993
- PMCID: PMC11687965
- DOI: 10.1152/ajpheart.00495.2022
Carotid dysfunction in senescent female mice is mediated by increased α1A-adrenoceptor activity and COX-derived vasoconstrictor prostanoids
Abstract
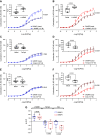
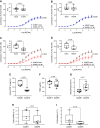
α-Adrenergic receptors are crucial regulators of vascular hemodynamics and essential pharmacological targets for cardiovascular diseases. With aging, there is an increase in sympathetic activation, which could contribute to the progression of aging-associated cardiovascular dysfunction, including stroke. Nevertheless, there is little information directly associating adrenergic receptor dysfunction in the blood vessels of aged females. This study determined the role of a-adrenergic receptors in carotid dysfunction of senescent female mice (accelerated-senescence prone, SAMP8), compared with a nonsenescent (accelerated-senescence prone, SAMR1). Vasoconstriction to phenylephrine (Phe) was markedly increased in common carotid artery of SAMP8 [area under the curve (AUC), 527 ± 53] compared with SAMR1 (AUC, 334 ± 30, P = 0.006). There were no changes in vascular responses to the vasoconstrictor agent U46619 or the vasodilators acetylcholine (ACh) and sodium nitroprusside (NPS). Hyperactivity to Phe in female SAMP8 was reduced by cyclooxygenase-1 and cyclooxygenase-2 inhibition and associated with augmented ratio of TXA2/PGI2 release (SAMR1, 1.1 ± 0.1 vs. SAMP8, 2.1 ± 0.3, P = 0.007). However, no changes in cyclooxygenase expression were seen in SAMP8 carotids. Selective α1A-receptor antagonism markedly reduced maximal contraction, whereas α1D antagonism induced a minor shift in Phe contraction in SAMP8 carotids. Ligand binding analysis revealed a threefold increase of α-adrenergic receptor density in smooth muscle cells (VSMCs) of SAMP8 vs. SAMR1. Phe rapidly increased intracellular calcium (Cai2+) in VSMCs via the α1A-receptor, with a higher peak in VSMCs from SAMP8. In conclusion, senescence intensifies vasoconstriction mediated by α1A-adrenergic signaling in the carotid of female mice by mechanisms involving increased Cai2+ and release of cyclooxygenase-derived prostanoids.NEW & NOTEWORTHY The present study provides evidence that senescence induces hyperreactivity of α1-adrenoceptor-mediated contraction of the common carotid. Impairment of α1-adrenoceptor responses is linked to increased Ca2+ influx and release of COX-derived vasoconstrictor prostanoids, contributing to carotid dysfunction in the murine model of female senescence (SAMP8). Increased reactivity of the common carotid artery during senescence may lead to morphological and functional changes in arteries of the cerebral microcirculation and contribute to cognitive decline in females. Because the elderly population is growing, elucidating the mechanisms of aging- and sex-associated vascular dysfunction is critical to better direct pharmacological and lifestyle interventions to prevent cardiovascular risk in both sexes.
Keywords: cerebrovascular function; common carotid; menopause; senescence-accelerated mice; α-adrenergic receptor.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Comment in
-
The intersection between senescence-mediated vascular dysfunction and cognitive impairment in female mice.Am J Physiol Heart Circ Physiol. 2023 Apr 1;324(4):H411-H413. doi: 10.1152/ajpheart.00076.2023. Epub 2023 Feb 17. Am J Physiol Heart Circ Physiol. 2023. PMID: 36800510 Free PMC article. No abstract available.
References
-
- El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention; and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation 142: e506–e532, 2020. doi:10.1161/CIR.0000000000000912. - DOI - PubMed
-
- Levine DA, Gross AL, Briceño EM, Tilton N, Giordani BJ, Sussman JB, Hayward RA, Burke JF, Hingtgen S, Elkind MSV, Manly JJ, Gottesman RF, Gaskin DJ, Sidney S, Sacco RL, Tom SE, Wright CB, Yaffe K, Galecki AT. Sex differences in cognitive decline among US adults. JAMA Netw Open 4: e210169, 2021. doi:10.1001/jamanetworkopen.2021.0169. - DOI - PMC - PubMed
-
- Ogola BO, Abshire CM, Visniauskas B, Kiley JX, Horton AC, Clark-Patterson GL, Kilanowski-Doroh I, Diaz Z, Bicego AN, McNally AB, Zimmerman MA, Groban L, Trask AJ, Miller KS, Lindsey SH. Sex differences in vascular aging and impact of GPER deletion. Am J Physiol Heart Circ Physiol 323: H336–H349, 2022. doi:10.1152/ajpheart.00238.2022. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

