Effectiveness of tailored COVID-19 messages for vulnerable Australians: A study protocol
- PMID: 36706131
- PMCID: PMC9882702
- DOI: 10.1371/journal.pone.0280865
Effectiveness of tailored COVID-19 messages for vulnerable Australians: A study protocol
Abstract
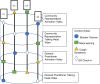
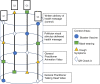
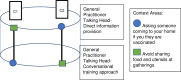
Multiple approaches can be used to communicate public health messages through mass media. It is unclear which approaches are superior for meeting the needs of the general community along with vulnerable population subgroups. To compare different public health strategy communication approaches for influencing the COVID-safe behavioural intentions of both community and vulnerable population subgroups. This study will conduct three concurrent 'helix' randomised controlled trials with Latin square sequencing and factorial intervention allocation to assess the effectiveness of different communication strategies amongst the Australian general community and six subgroups that are considered vulnerable to contracting, transmitting or experiencing severe consequences of COVID-19 infection. Communication approaches being compared include: the format of communication (written versus video), who is providing information (general practitioner, politician, community-representative), what is said and how it is delivered (direct information provision versus conversational approach) and the visual content of video messaging (animation versus 'talking head'). Recruited participants will be randomly allocated to receive a specific combination of health messaging strategies using six different COVID-19 context areas. Outcomes will be assessed in a survey using behaviour intention questions, and questions surrounding level of agreement with feeling represented in the health messaging strategy. These trials will use a unique research approach to provide an experimental evidence base to help guide development of impactful and inclusive COVID-19 and related public health messaging. All three trials are registered with the Australian New Zealand Clinical Trials Registry (ANZCTR). Trial 1: Update and impact of Government recommendations about COVID-19 (coronavirus)-Stage 3, Trial 1, vulnerable subgroup populations (ACTRN12622000606785). Trial 2: Update and impact of Government recommendations about COVID-19 (coronavirus)-Stage 3, Trial 2, community group (ACTRN12622000605796). Trial 3: Update and impact of Government recommendations about COVID-19 (coronavirus)-Stage 3, Trial 3, What communication strategy is most effective for both vulnerable and community group populations? (ACTRN12622000617763).
Copyright: © 2023 Jepson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
The effect of framing and communicating COVID-19 vaccine side-effect risks on vaccine intentions for adults in the UK and the USA: A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Sep 6;22(1):592. doi: 10.1186/s13063-021-05484-2. Trials. 2021. PMID: 34488843 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Testing an early online intervention for the treatment of disturbed sleep during the COVID-19 pandemic (Sleep COVID-19): structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Aug 8;21(1):704. doi: 10.1186/s13063-020-04644-0. Trials. 2020. PMID: 32771068 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
Cited by
-
Navigating the COVID-19 pandemic: Experiences and self-management approaches adopted by people with interstitial lung disease.Chron Respir Dis. 2024 Jan-Dec;21:14799731231226236. doi: 10.1177/14799731231226236. Chron Respir Dis. 2024. PMID: 38193428 Free PMC article.
References
-
- Australian Government Department of Health [Internet]. Coronavirus (COVID-19) resources for the general public; c2022. [cited 2022 July 15]. Available from: https://www.health.gov.au/resources/collections/novel-coronavirus-2019-n....
-
- World Health Organization; [Internet]. Coronavirus disease (COVID-19) news; c2022. [cited 2022 July 15]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/media-re....
-
- Jones B, Woolfenden S, Pengilly S, Breen C, Cohn R, Biviano L, et al.. COVID‐19 pandemic: The impact on vulnerable children and young people in Australia. Journal of Paediatrics and Child Health. 2020;56(12):1851–5. - PubMed
-
- Calderón-Larrañaga A, Vetrano DL, Rizzuto D, Bellander T, Fratiglioni L, Dekhtyar S. High excess mortality in areas with young and socially vulnerable populations during the COVID-19 outbreak in Stockholm Region, Sweden. British Medical Journal Global Health. 2020;5(10):e003595. doi: 10.1136/bmjgh-2020-003595 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

