T-independent responses to polysaccharides in humans mobilize marginal zone B cells prediversified against gut bacterial antigens
- PMID: 36706172
- PMCID: PMC7614366
- DOI: 10.1126/sciimmunol.ade1413
T-independent responses to polysaccharides in humans mobilize marginal zone B cells prediversified against gut bacterial antigens
Abstract
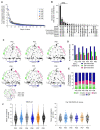
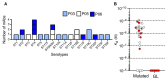
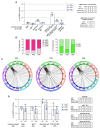
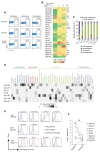
Marginal zone (MZ) B cells are one of the main actors of T-independent (TI) responses in mice. To identify the B cell subset(s) involved in such responses in humans, we vaccinated healthy individuals with Pneumovax, a model TI vaccine. By high-throughput repertoire sequencing of plasma cells (PCs) isolated 7 days after vaccination and of different B cell subpopulations before and after vaccination, we show that the PC response mobilizes large clones systematically, including an immunoglobulin M component, whose diversification and amplification predated the pneumococcal vaccination. These clones could be mainly traced back to MZ B cells, together with clonally related IgA+ and, to a lesser extent, IgG+CD27+ B cells. Recombinant monoclonal antibodies isolated from large PC clones recognized a wide array of bacterial species from the gut flora, indicating that TI responses in humans largely mobilize MZ and switched B cells that most likely prediversified during mucosal immune responses against bacterial antigens and acquired pneumococcal cross-reactivity through somatic hypermutation.
Conflict of interest statement
Figures



Comment in
-
Weller et al., T-independent responses to polysaccharides in humans mobilize marginal zone B cells pre-diversified against gut bacterial antigens.Immunol Lett. 2023 Apr-May;256-257:66-67. doi: 10.1016/j.imlet.2023.04.004. Epub 2023 Apr 9. Immunol Lett. 2023. PMID: 37040847 No abstract available.
References
-
- Mosier DE, Mond JJ, Goldings EA. The ontogeny of thymic independent antibody responses in vitro in normal mice and mice with an X-linked B cell defect. J Immunol. 1977;119:1874–1878. - PubMed
-
- Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. - PubMed
-
- Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349–354. - PubMed
-
- Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. - PubMed
-
- Balázs M, Martin F, Zhou T, Kearney JF. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

