Conserved meiotic mechanisms in the cnidarian Clytia hemisphaerica revealed by Spo11 knockout
- PMID: 36706182
- PMCID: PMC9882977
- DOI: 10.1126/sciadv.add2873
Conserved meiotic mechanisms in the cnidarian Clytia hemisphaerica revealed by Spo11 knockout
Abstract
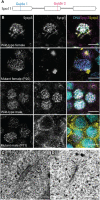
During meiosis, DNA recombination allows the shuffling of genetic information between the maternal and paternal chromosomes. Recombination is initiated by double-strand breaks (DSBs) catalyzed by the conserved enzyme Spo11. How this crucial event is connected to other meiotic processes is unexpectedly variable depending on the species. Here, we knocked down Spo11 by CRISPR in the jellyfish Clytia hemisphaerica. Germ cells in Clytia Spo11 mutants fail to assemble synaptonemal complexes and chiasmata, and in consequence, homologous chromosome pairs in females remain unassociated during oocyte growth and meiotic divisions, creating aneuploid but fertilizable eggs that develop into viable larvae. Clytia thus shares an ancient eukaryotic dependence of synapsis and chromosome segregation on Spo11-generated DSBs. Phylogenetically, Clytia belongs to Cnidaria, the sister clade to Bilateria where classical animal model species are found, so these results provide fresh evolutionary perspectives on meiosis regulation.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources

