Chemotherapy-Associated Thrombotic Microangiopathy
- PMID: 36706238
- PMCID: PMC10103319
- DOI: 10.34067/KID.0000000000000061
Chemotherapy-Associated Thrombotic Microangiopathy
Abstract
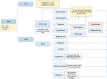
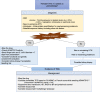
Thrombotic microangiopathy (TMA) is a syndrome of microangiopathic hemolytic anemia and thrombocytopenia with end-organ dysfunction. Although the advent of plasma exchange, immunosuppression, and complement inhibition has improved morbidity and mortality for primary TMAs, the management of secondary TMAs, particularly drug-induced TMA, remains less clear. TMA related to cancer drugs disrupts the antineoplastic treatment course, increasing the risk of cancer progression. Chemotherapeutic agents such as mitomycin-C, gemcitabine, and platinum-based drugs as well as targeted therapies such as antiangiogenesis agents and proteasome inhibitors have been implicated in oncotherapy-associated TMA. Among TMA subtypes, drug-induced TMA is less well-understood. Treatment generally involves withdrawal of the offending agent and supportive care targeting blood pressure and proteinuria reduction. Immunosuppression and therapeutic plasma exchange have not shown clear benefit. The terminal complement inhibitor, eculizumab, has shown promising results in some cases of chemotherapy-associated TMA including in re-exposure. However, the data are limited, and unlike in primary atypical hemolytic uremic syndrome, the role of complement in the pathogenesis of drug-induced TMA is unclear. Larger multicenter studies and unified definitions are needed to elucidate the extent of the problem and potential treatment strategies.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Figures

Similar articles
-
Microangiopathy in Cancer: Causes, Consequences, and Management.Cancer Treat Res. 2019;179:151-158. doi: 10.1007/978-3-030-20315-3_10. Cancer Treat Res. 2019. PMID: 31317486
-
Thrombotic Microangiopathy Syndromes-Common Ground and Distinct Frontiers.Adv Chronic Kidney Dis. 2022 Mar;29(2):149-160.e1. doi: 10.1053/j.ackd.2021.11.006. Adv Chronic Kidney Dis. 2022. PMID: 35817522 Review.
-
Thrombotic microangiopathy with targeted cancer agents.Clin Cancer Res. 2011 Sep 15;17(18):5858-66. doi: 10.1158/1078-0432.CCR-11-0804. Epub 2011 Aug 3. Clin Cancer Res. 2011. PMID: 21813634 Free PMC article. Review.
-
A review of thrombotic microangiopathies in multiple myeloma.Leuk Res. 2019 Oct;85:106195. doi: 10.1016/j.leukres.2019.106195. Epub 2019 Jul 29. Leuk Res. 2019. PMID: 31404728 Review.
-
Thrombotic Microangiopathy in Cancer.Semin Thromb Hemost. 2019 Jun;45(4):348-353. doi: 10.1055/s-0039-1687893. Epub 2019 Apr 30. Semin Thromb Hemost. 2019. PMID: 31041804 Review.
Cited by
-
Eculizumab is efficacious and safe in pediatric patients with various forms of hemolytic uremic syndrome: a retrospective clinical experience of a tertiary center.Front Pharmacol. 2025 Apr 4;16:1535407. doi: 10.3389/fphar.2025.1535407. eCollection 2025. Front Pharmacol. 2025. PMID: 40255570 Free PMC article.
-
The Phenomenon of Thrombotic Microangiopathy in Cancer Patients.Int J Mol Sci. 2024 Aug 21;25(16):9055. doi: 10.3390/ijms25169055. Int J Mol Sci. 2024. PMID: 39201740 Free PMC article. Review.
-
Chemotherapy-induced acute kidney injury: epidemiology, pathophysiology, and therapeutic approaches.Front Nephrol. 2024 Aug 9;4:1436896. doi: 10.3389/fneph.2024.1436896. eCollection 2024. Front Nephrol. 2024. PMID: 39185276 Free PMC article. Review.
-
Frequency and characteristics of chemotherapy-associated thrombotic microangiopathy: Analysis from a large pharmacovigilance database.Am J Hematol. 2023 Dec;98(12):E369-E372. doi: 10.1002/ajh.27101. Epub 2023 Sep 23. Am J Hematol. 2023. PMID: 37740927 Free PMC article.
-
SUrvey of renal Biopsy registry database and Anticancer dRUg therapy in Japan (SUBARU-J study).Clin Kidney J. 2024 Nov 28;17(12):sfae327. doi: 10.1093/ckj/sfae327. eCollection 2024 Dec. Clin Kidney J. 2024. PMID: 39664993 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

