Development in the Concept of Bacterial Polysaccharide Repeating Unit-Based Antibacterial Conjugate Vaccines
- PMID: 36706246
- PMCID: PMC9930202
- DOI: 10.1021/acsinfecdis.2c00559
Development in the Concept of Bacterial Polysaccharide Repeating Unit-Based Antibacterial Conjugate Vaccines
Abstract
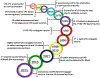
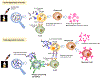
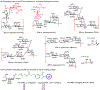
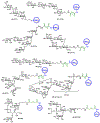
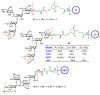
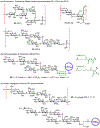
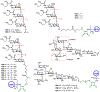
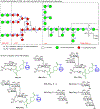
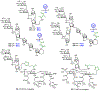
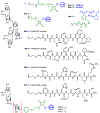
The surface of cells is coated with a dense layer of glycans, known as the cell glycocalyx. The complex glycans in the glycocalyx are involved in various biological events, such as bacterial pathogenesis, protection of bacteria from environmental stresses, etc. Polysaccharides on the bacterial cell surface are highly conserved and accessible molecules, and thus they are excellent immunological targets. Consequently, bacterial polysaccharides and their repeating units have been extensively studied as antigens for the development of antibacterial vaccines. This Review surveys the recent developments in the synthetic and immunological investigations of bacterial polysaccharide repeating unit-based conjugate vaccines against several human pathogenic bacteria. The major challenges associated with the development of functional carbohydrate-based antibacterial conjugate vaccines are also considered.
Keywords: Antibacterial Vaccine; Conjugate Vaccine; Glycoconjugate; Immunogenicity; Oligosaccharide; Polysaccharide.
Figures

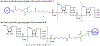
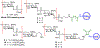
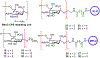
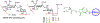
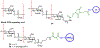
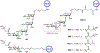





Similar articles
-
Analysis of immunogenicity and purification methods in conjugated polysaccharide vaccines: a new approach in fighting pathogenic bacteria.Front Immunol. 2024 Nov 20;15:1483740. doi: 10.3389/fimmu.2024.1483740. eCollection 2024. Front Immunol. 2024. PMID: 39635523 Free PMC article. Review.
-
Differential regulation of polysaccharide-specific antibody responses to isolated polysaccharides, conjugate vaccines, and intact Gram-positive versus Gram-negative extracellular bacteria.Vaccine. 2016 Jun 24;34(30):3542-8. doi: 10.1016/j.vaccine.2015.12.077. Epub 2016 Apr 29. Vaccine. 2016. PMID: 27133879 Review.
-
Synthetic carbohydrate-based vaccines: challenges and opportunities.J Biomed Sci. 2020 Jan 3;27(1):9. doi: 10.1186/s12929-019-0591-0. J Biomed Sci. 2020. PMID: 31900143 Free PMC article. Review.
-
Advancing Homogeneous Antimicrobial Glycoconjugate Vaccines.Acc Chem Res. 2017 May 16;50(5):1270-1279. doi: 10.1021/acs.accounts.7b00106. Epub 2017 May 2. Acc Chem Res. 2017. PMID: 28463499
-
Glycan Microarrays Containing Synthetic Streptococcus pneumoniae CPS Fragments and Their Application to Vaccine Development.Methods Mol Biol. 2022;2460:193-206. doi: 10.1007/978-1-0716-2148-6_12. Methods Mol Biol. 2022. PMID: 34972938
Cited by
-
Unveiling the Group A Streptococcus Vaccine-Based L-Rhamnose from Backbone of Group A Carbohydrate: Current Insight Against Acute Rheumatic Fever to Reduce the Global Burden of Rheumatic Heart Disease.F1000Res. 2025 Jan 30;13:132. doi: 10.12688/f1000research.144903.3. eCollection 2024. F1000Res. 2025. PMID: 39959434 Free PMC article. Review.
-
Analysis of immunogenicity and purification methods in conjugated polysaccharide vaccines: a new approach in fighting pathogenic bacteria.Front Immunol. 2024 Nov 20;15:1483740. doi: 10.3389/fimmu.2024.1483740. eCollection 2024. Front Immunol. 2024. PMID: 39635523 Free PMC article. Review.
-
A Fluorinated Sialic Acid Vaccine Lead Against Meningitis B and C.J Am Chem Soc. 2024 Jun 5;146(22):15366-15375. doi: 10.1021/jacs.4c03179. Epub 2024 May 20. J Am Chem Soc. 2024. PMID: 38768956 Free PMC article.
-
Effect of Side-Chain Functional Groups in the Immunogenicity of Bacterial Surface Glycans.Molecules. 2023 Oct 16;28(20):7112. doi: 10.3390/molecules28207112. Molecules. 2023. PMID: 37894591 Free PMC article. Review.
-
Identification and Characterization of the Two Glycosyltransferases Required for the Polymerization of the HS:1 Serotype Capsular Polysaccharide of Campylobacter jejuni G1.Biochemistry. 2025 Mar 18;64(6):1370-1379. doi: 10.1021/acs.biochem.4c00803. Epub 2025 Feb 28. Biochemistry. 2025. PMID: 40020177 Free PMC article.
References
-
- Doron S; Gorbach SL Bacterial Infections: Overview. In International Encyclopedia of Public Health, Heggenhougen HK Ed.; Academic Press, 2008; pp 273–282.
-
- Smallpox overview. 2004, Pamphlet (or booklet).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

