Different routes of MHC-I delivery to phagosomes and their consequences to CD8 T cell immunity
- PMID: 36706521
- PMCID: PMC10023361
- DOI: 10.1016/j.smim.2023.101713
Different routes of MHC-I delivery to phagosomes and their consequences to CD8 T cell immunity
Abstract
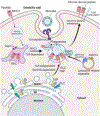
Dendritic cells (DCs) present internalized antigens to CD8 T cells through cross-presentation by major histocompatibility complex class I (MHC-I) molecules. While conventional cDC1 excel at cross-presentation, cDC2 can be licensed to cross-present during infection by signals from inflammatory receptors, most prominently Toll-like receptors (TLRs). At the core of the regulation of cross-presentation by TLRs is the control of subcellular MHC-I traffic. Within DCs, MHC-I are enriched within endosomal recycling compartments (ERC) and traffic to microbe-carrying phagosomes under the control of phagosome-compartmentalized TLR signals to favor CD8 T cell cross-priming to microbial antigens. Viral blockade of the transporter associated with antigen processing (TAP), known to inhibit the classic MHC-I presentation of cytoplasmic protein-derived peptides, depletes the ERC stores of MHC-I to simultaneously also block TLR-regulated cross-presentation. DCs counter this impairment in the two major pathways of MHC-I presentation to CD8 T cells by mobilizing noncanonical cross-presentation, which delivers MHC-I to phagosomes from a new location in the ER-Golgi intermediate compartment (ERGIC) where MHC-I abnormally accumulate upon TAP blockade. Noncanonical cross-presentation thus rescues MHC-I presentation and cross-primes TAP-independent CD8 T cells best-matched against target cells infected with immune evasive viruses. Because noncanonical cross-presentation relies on a phagosome delivery route of MHC-I that is not under TLR control, it risks potential cross-presentation of self-antigens during infection. Here I review these findings to illustrate how the subcellular route of MHC-I to phagosomes critically impacts the regulation of cross-presentation and the nature of the CD8 T cell response to infection and cancer. I highlight important and novel implications to CD8 T cell vaccines and immunotherapy.
Keywords: CD8 T cells; Cross-presentation; Dendritic cells; Noncanonical cross-presentation; Phagosomes; Toll-like receptors; Vesicular traffic.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest The author declares no financial conflict.
Figures

Similar articles
-
TAP dysfunction in dendritic cells enables noncanonical cross-presentation for T cell priming.Nat Immunol. 2021 Apr;22(4):497-509. doi: 10.1038/s41590-021-00903-7. Epub 2021 Mar 31. Nat Immunol. 2021. PMID: 33790474 Free PMC article.
-
TLR signals induce phagosomal MHC-I delivery from the endosomal recycling compartment to allow cross-presentation.Cell. 2014 Jul 31;158(3):506-21. doi: 10.1016/j.cell.2014.04.054. Cell. 2014. PMID: 25083866 Free PMC article.
-
The show and tell of cross-presentation.Adv Immunol. 2023;159:33-114. doi: 10.1016/bs.ai.2023.08.002. Epub 2023 Oct 12. Adv Immunol. 2023. PMID: 37996207 Review.
-
The comings and goings of MHC class I molecules herald a new dawn in cross-presentation.Immunol Rev. 2016 Jul;272(1):65-79. doi: 10.1111/imr.12428. Immunol Rev. 2016. PMID: 27319343 Free PMC article. Review.
-
TAP-ing into the cross-presentation secrets of dendritic cells.Curr Opin Immunol. 2023 Aug;83:102327. doi: 10.1016/j.coi.2023.102327. Epub 2023 Apr 26. Curr Opin Immunol. 2023. PMID: 37116384 Review.
Cited by
-
The Atlantic Cod MHC I compartment has the properties needed for cross-presentation in the absence of MHC II.Sci Rep. 2024 Oct 25;14(1):25404. doi: 10.1038/s41598-024-76225-z. Sci Rep. 2024. PMID: 39455705 Free PMC article.
-
Zinc-Organometallic Framework Vaccine Controlled-Release Zn2+ Regulates Tumor Extracellular Matrix Degradation Potentiate Efficacy of Immunotherapy.Adv Sci (Weinh). 2023 Sep;10(27):e2302967. doi: 10.1002/advs.202302967. Epub 2023 Jul 13. Adv Sci (Weinh). 2023. PMID: 37439462 Free PMC article.
-
Strategies to overcome low MHC-I expression in paediatric and adult tumours.Immunother Adv. 2023 Dec 11;4(1):ltad028. doi: 10.1093/immadv/ltad028. eCollection 2024. Immunother Adv. 2023. PMID: 38223409 Free PMC article. Review.
-
Novel therapeutic strategies and recent advances in gut microbiota synergy with nanotechnology for colorectal cancer treatment.Mater Today Bio. 2025 Feb 20;31:101601. doi: 10.1016/j.mtbio.2025.101601. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40066079 Free PMC article. Review.
-
Molecular Characteristics, Functional Definitions, and Regulatory Mechanisms for Cross-Presentation Mediated by the Major Histocompatibility Complex: A Comprehensive Review.Int J Mol Sci. 2023 Dec 22;25(1):196. doi: 10.3390/ijms25010196. Int J Mol Sci. 2023. PMID: 38203367 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

