Accelerated waning of immune responses to a third COVID-19 vaccination in patients with immune-mediated inflammatory diseases
- PMID: 36706534
- PMCID: PMC9771756
- DOI: 10.1016/j.jaut.2022.102981
Accelerated waning of immune responses to a third COVID-19 vaccination in patients with immune-mediated inflammatory diseases
Abstract
Background: A 3rd COVID-19 vaccination is currently recommended for patients under immunosuppression. However, a fast decline of antibodies against the SARS-CoV-2 receptor-binding domain (RBD) of the spike protein has been observed. Currently it remains unclear whether immunosuppressive therapy affects kinetics of humoral and cellular immune responses.
Methods: 50 patients under immunosuppression and 42 healthy controls (HCs) received a 3rd dose of an mRNA-based vaccine and were monitored over a 12-weeks period. Humoral immune response was assessed 4 and 12 weeks after 3rd dose. Antibodies were quantified using the Elecsys Anti-SARS-CoV-2 Spike immunoassay against the receptor-binding domain (RBD) of the spike protein. SARS-CoV-2-specific T cell responses were quantified by IFN-γ ELISpot assays. Adverse events, including SARS-CoV-2 infections, were monitored over a 12-week period.
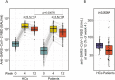
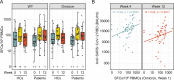
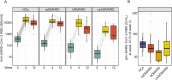
Results: At week 12, reduced anti-RBD antibody levels were observed in IMID patients as compared to HCs (median antibody level 5345 BAU/ml [1781-10,208] versus 9650 BAU/ml [6633-16,050], p < 0.001). Reduction in relative antibody levels was significantly higher in IMID patients as compared to HCs at week 12 (p < 0.001). Lowest anti-RBD antibody levels were detected in IMID patients who received biological disease-modifying anti-rheumatic drugs (DMARDs) or a combination therapy with conventional synthetic and biological DMARDs. Number of SARS-CoV-2-specific T cells against wildtype and Omicron variants remained stable over 12 weeks in IMID patients. No serious adverse events were reported.
Conclusion: Due to a fast decline in anti-RBD antibodies in IMID patients an early 4th vaccination should be considered in this vulnerable group of patients.
Keywords: COVID-19; Immunosuppression; SARS-CoV-2; Vaccination.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest DM reports support for meeting attendances from Pfizer and consultation fees from AstraZeneca; JS reports about grants, consulting and personal fees from AbbVie, Amgen, AstraZeneca, Astro, Bristol-Myers Squibb, Celltrion, Gilead-Galapagos, Janssen, Lilly, Pfizer, R-Pharma, Samsung, Sanofi, Chugai, Merck Sharp & Dohme, Novartis-Sandoz Roche, Samsung and UCB and grants from Abbvie, AstraZeneca, Lilly, Novartis, and Roche; HR received speaker fees from Gilead, Merck Sharp and Pfizer and travel support from Janssen; HH received grants from Glock Health, BlueSky Immunotherapies and Neutrolis; DA received grants, speaker fees, or consultancy fees from Abbvie, Amgen, Galapagos, Lilly, Janssen, Merck, Novartis, Pfizer, Sandoz, and Sanofi; RK reports consulting fees from AstraZeneca, Takeda Pharma, MEDahead and Janssen Cilag and speaker fees from Otsuka; ES received travel support from Pfizer, Bristol-Myers Squibb and Boehringer-Ingelheim and speaker fees from Lilly; MB reports about personal fees from Eli-Lilly. All other authors declare no competing interests.
Figures
Similar articles
-
The persistence of anti-Spike antibodies following two SARS-CoV-2 vaccine doses in patients on immunosuppressive therapy compared to healthy controls-a prospective cohort study.BMC Med. 2022 Oct 5;20(1):378. doi: 10.1186/s12916-022-02587-8. BMC Med. 2022. PMID: 36199139 Free PMC article.
-
Kinetics of the B- and T-Cell Immune Responses After 6 Months From SARS-CoV-2 mRNA Vaccination in Patients With Rheumatoid Arthritis.Front Immunol. 2022 Feb 28;13:846753. doi: 10.3389/fimmu.2022.846753. eCollection 2022. Front Immunol. 2022. PMID: 35309297 Free PMC article.
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity.Ann Rheum Dis. 2021 Oct;80(10):1345-1350. doi: 10.1136/annrheumdis-2021-220781. Epub 2021 Jul 20. Ann Rheum Dis. 2021. PMID: 34285048
-
Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: a randomised, double-blind, placebo-controlled, phase 1 trial.Lancet Microbe. 2022 Mar;3(3):e193-e202. doi: 10.1016/S2666-5247(21)00280-9. Epub 2022 Jan 24. Lancet Microbe. 2022. PMID: 35098177 Free PMC article. Clinical Trial.
Cited by
-
The Kinetics of Humoral and Cellular Responses after the Booster Dose of COVID-19 Vaccine in Inflammatory Arthritis Patients.Viruses. 2023 Feb 24;15(3):620. doi: 10.3390/v15030620. Viruses. 2023. PMID: 36992329 Free PMC article.
-
Uptake, effectiveness and safety of COVID-19 vaccines in individuals at clinical risk due to immunosuppressive drug therapy or transplantation procedures: a population-based cohort study in England.BMC Med. 2024 Jun 10;22(1):237. doi: 10.1186/s12916-024-03457-1. BMC Med. 2024. PMID: 38858672 Free PMC article.
-
Did We Overreact? Insights on COVID-19 Disease and Vaccination in a Large Cohort of Immune-Mediated Inflammatory Disease Patients during Sequential Phases of the Pandemic (The BELCOMID Study).Vaccines (Basel). 2024 Oct 11;12(10):1157. doi: 10.3390/vaccines12101157. Vaccines (Basel). 2024. PMID: 39460324 Free PMC article.
-
Biological and glucocorticoids treatment impair the medium-term immunogenicity to SARS-CoV-2 mRNA vaccines in autoimmune inflammatory rheumatic diseases.Eur J Med Res. 2024 Jan 5;29(1):28. doi: 10.1186/s40001-023-01620-7. Eur J Med Res. 2024. PMID: 38183092 Free PMC article.
References
-
- Furer V., Eviatar T., Zisman D., Peleg H., Paran D., Levartovsky D., Zisapel M., Elalouf O., Kaufman I., Meidan R., Broyde A., Polachek A., Wollman J., Litinsky I., Meridor K., Nochomovitz H., Silberman A., Rosenberg D., Feld J., Haddad A., Gazzit T., Elias M., Higazi N., Kharouf F., Shefer G., Sharon O., Pel S., Nevo S., Elkayam O. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann. Rheum. Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. - DOI - PubMed
-
- Wieske L., van Dam K.P.J., Steenhuis M., Stalman E.W., Kummer L.Y.L., van Kempen Z.L.E., Killestein J., Volkers A.G., Tas S.W., Boekel L., Wolbink G.J., van der Kooi A.J., Raaphorst J., Löwenberg M., Takkenberg R.B., D'Haens G.R.A.M., Spuls P.I., Bekkenk M.W., Musters A.H., Post N.F., Bosma A.L., Hilhorst M.L., Vegting Y., Bemelman F.J., Voskuyl A.E., Broens B., Sanchez A.P., van Els C.A.C.M., de Wit J., Rutgers A., de Leeuw K., Horváth B., Verschuuren J.J.G.M., Ruiter A.M., van Ouwerkerk L., van der Woude D., Allaart R.C.F., Teng Y.K.O., van Paassen P., Busch M.H., Jallah P.B.P., Brusse E., van Doorn P.A., Baars A.E., Hijnen D.J., Schreurs C.R.G., van der Pol W.L., Goedee H.S., Keijzer S., Keijser J.B.D., Boogaard A., Cristianawati O., Ten Brinke A., Verstegen N.J.M., Zwinderman K.A.H., van Ham S.M., Kuijpers T.W., Rispens T., Eftimov F. T2B! Immunity against SARS-CoV-2 study group, Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022 doi: 10.1016/S2665-9913(22)00034-0. - DOI - PMC - PubMed
-
- Simon D., Tascilar K., Fagni F., Kleyer A., Krönke G., Meder C., Dietrich P., Orlemann T., Mößner J., Taubmann J., Mutlu M.Y., Knitza J., Kemenes S., Liphardt A.-M., Schönau V., Bohr D., Schuster L., Hartmann F., Minopoulou I., Leppkes M., Ramming A., Pachowsky M., Schuch F., Ronneberger M., Kleinert S., Hueber A.J., Manger K., Manger B., Atreya R., Berking C., Sticherling M., Neurath M.F., Schett G. Intensity and longevity of SARS-CoV-2 vaccination response in patients with immune-mediated inflammatory disease: a prospective cohort study. Lancet Rheumatol. 2022;4 doi: 10.1016/S2665-9913(22)00191-6. e614–e625. - DOI - PMC - PubMed
-
- Haberman R.H., Herati R., Simon D., Samanovic M., Blank R.B., Tuen M., Koralov S.B., Atreya R., Tascilar K., Allen J.R., Castillo R., Cornelius A.R., Rackoff P., Solomon G., Adhikari S., Azar N., Rosenthal P., Izmirly P., Samuels J., Golden B., Reddy S.M., Neurath M.F., Abramson S.B., Schett G., Mulligan M.J., Scher J.U. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann. Rheum. Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

