Maternal immune activation alters social affective behavior and sensitivity to corticotropin releasing factor in male but not female rats
- PMID: 36706685
- PMCID: PMC9974777
- DOI: 10.1016/j.yhbeh.2023.105313
Maternal immune activation alters social affective behavior and sensitivity to corticotropin releasing factor in male but not female rats
Abstract
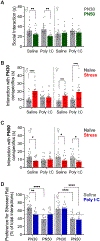
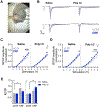
Prenatal infection increases risk for neurodevelopmental disorders such as autism in offspring. In rodents, prenatal administration of the viral mimic Polyinosinic: polycytidylic acid (Poly I: C) allows for investigation of developmental consequences of gestational sickness on offspring social behavior and neural circuit function. Because maternal immune activation (MIA) disrupts cortical development and sociability, we examined approach and avoidance in a rat social affective preference (SAP) task. Following maternal Poly I:C (0.5 mg/kg) injection on gestational day 12.5, male adult offspring (PN 60-64) exhibited atypical social interactions with stressed conspecifics whereas female SAP behavior was unaffected by maternal Poly I:C. Social responses to stressed conspecifics depend upon the insular cortex where corticotropin releasing factor (CRF) modulates synaptic transmission and SAP behavior. We characterized insular field excitatory postsynaptic potentials (fEPSP) in adult offspring of Poly I:C or control treated dams. Male MIA offspring showed decreased sensitivity to CRF (300 nM) while female MIA offspring showed greater sensitivity to CRF compared to sham offspring. These sex specific effects appear to be behaviorally relevant as CRF injected into the insula of male and female rats prior to social exploration testing had no effect in MIA male offspring but increased social interaction in female MIA offspring. We examined the cellular distribution of CRF receptor mRNA but found no effect of maternal Poly I:C in the insula. Together, these experiments reveal sex specific effects of prenatal infection on offspring responses to social affective stimuli and identify insular CRF signaling as a novel neurobiological substrate for autism risk.
Keywords: Corticotropin releasing factor; Insular cortex; Rat; Social affect.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures


Similar articles
-
Insular cortex corticotropin-releasing factor integrates stress signaling with social affective behavior.Neuropsychopharmacology. 2022 May;47(6):1156-1168. doi: 10.1038/s41386-022-01292-7. Epub 2022 Feb 26. Neuropsychopharmacology. 2022. PMID: 35220413 Free PMC article.
-
Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring.Biol Psychiatry. 2014 Feb 15;75(4):332-41. doi: 10.1016/j.biopsych.2013.06.025. Epub 2013 Sep 5. Biol Psychiatry. 2014. PMID: 24011823 Free PMC article.
-
Maternal Immune Activation during Pregnancy Alters Postnatal Brain Growth and Cognitive Development in Nonhuman Primate Offspring.J Neurosci. 2021 Dec 1;41(48):9971-9987. doi: 10.1523/JNEUROSCI.0378-21.2021. Epub 2021 Oct 4. J Neurosci. 2021. PMID: 34607967 Free PMC article.
-
The Outcomes of Maternal Immune Activation Induced with the Viral Mimetic Poly I:C on Microglia in Exposed Rodent Offspring.Dev Neurosci. 2023;45(4):191-209. doi: 10.1159/000530185. Epub 2023 Mar 21. Dev Neurosci. 2023. PMID: 36944325 Review.
-
Short- and long-term consequences of corticotropin-releasing factor in early development.Ann N Y Acad Sci. 1999;897:76-91. doi: 10.1111/j.1749-6632.1999.tb07880.x. Ann N Y Acad Sci. 1999. PMID: 10676437 Review.
Cited by
-
Maternal immune activation with toll-like receptor 7 agonist during mid-gestation alters juvenile and adult developmental milestones and behavior.J Neuroendocrinol. 2024 Aug;36(8):e13417. doi: 10.1111/jne.13417. Epub 2024 Jun 1. J Neuroendocrinol. 2024. PMID: 38822791 Free PMC article.
-
Prenatal and postnatal neuroimmune interactions in neurodevelopmental disorders.Nat Immunol. 2024 Apr;25(4):598-606. doi: 10.1038/s41590-024-01797-x. Epub 2024 Apr 2. Nat Immunol. 2024. PMID: 38565970 Review.
References
-
- Arakawa H, Cruz S, Deak T, 2011. From models to mechanisms: odorant communication as a key determinant of social behavior in rodents during illness-associated states. Neurosci. Biobehav. Rev, Pioneering Research in Affective Neuroscience: Celebrating the Work of Dr. Jaak Panksepp 35, 1916–1928. 10.1016/j.neubiorev.2011.03.007. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

