Local 5-HT signaling bi-directionally regulates the coincidence time window for associative learning
- PMID: 36706757
- PMCID: PMC11152601
- DOI: 10.1016/j.neuron.2022.12.034
Local 5-HT signaling bi-directionally regulates the coincidence time window for associative learning
Abstract
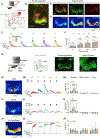
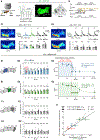
The coincidence between conditioned stimulus (CS) and unconditioned stimulus (US) is essential for associative learning; however, the mechanism regulating the duration of this temporal window remains unclear. Here, we found that serotonin (5-HT) bi-directionally regulates the coincidence time window of olfactory learning in Drosophila and affects synaptic plasticity of Kenyon cells (KCs) in the mushroom body (MB). Utilizing GPCR-activation-based (GRAB) neurotransmitter sensors, we found that KC-released acetylcholine (ACh) activates a serotonergic dorsal paired medial (DPM) neuron, which in turn provides inhibitory feedback to KCs. Physiological stimuli induce spatially heterogeneous 5-HT signals, which proportionally gate the intrinsic coincidence time windows of different MB compartments. Artificially reducing or increasing the DPM neuron-released 5-HT shortens or prolongs the coincidence window, respectively. In a sequential trace conditioning paradigm, this serotonergic neuromodulation helps to bridge the CS-US temporal gap. Altogether, we report a model circuitry for perceiving the temporal coincidence and determining the causal relationship between environmental events.
Keywords: GRAB sensor; associative learning; coincidence time window; olfaction; serotonin; synaptic plasticity.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





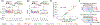
References
-
- Bernstein AL (1934). Temporal factors in the formation of conditioned eyelid reactions in human subjects. J. Gen. Psychol 10, 173–197.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

