Gut Microbiota-Derived Glutamine Attenuates Liver Ischemia/Reperfusion Injury via Macrophage Metabolic Reprogramming
- PMID: 36706918
- PMCID: PMC10140379
- DOI: 10.1016/j.jcmgh.2023.01.004
Gut Microbiota-Derived Glutamine Attenuates Liver Ischemia/Reperfusion Injury via Macrophage Metabolic Reprogramming
Abstract
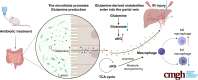
Background & aims: Many studies have revealed crucial roles of the gut microbiota and its metabolites in liver disease progression. However, the mechanism underlying their effects on liver ischemia/reperfusion (I/R) injury remain largely unknown. Here, we investigate the function of gut microbiota and its metabolites in liver I/R injury.
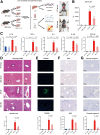
Methods: C57BL/6 mice was pretreated with an antibiotic cocktail. Then, we used multi-omics detection methods including 16s rRNA sequencing, ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) to explore the changes of gut microbiota and metabolites in both feces and portal blood to reveal the mechanism of their protective effect in liver I/R injury.
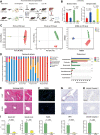
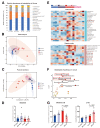
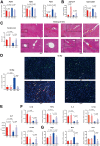
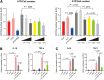
Results: We found that antibiotic pretreatment (ABX) could significantly reduce the severity of I/R-induced hepatic injury, and this effect could be transferred to germ-free mice by fecal microbiota transplantation (FMT), suggesting a protective role of the gut microbiota depletion. During I/R, the rates of serum α-ketoglutarate (αKG) production and glutamate reduction, downstream products of gut microbiota-derived glutamine, were more significant in the ABX mice. Then, we showed that αKG could promote alternative (M2) macrophage activation through oxidative phosphorylation, and oligomycin A could inhibit M2 macrophage polarization and reversed this protective effect.
Conclusions: These findings show that the gut microbiota and its metabolites play critical roles in hepatic I/R injury by modulating macrophage metabolic reprogramming. Potential therapies that target macrophage metabolism, including antibiotic therapies and novel immunometabolism modulators, can be exploited for the treatment of liver I/R injury.
Keywords: Glutamine; Immunometabolism; Liver Ischemia/Reperfusion Injury; Macrophage Reprogramming; Microbiota; a-ketoglutarate.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Gut microbiota-derived succinate aggravates acute lung injury after intestinal ischaemia/reperfusion in mice.Eur Respir J. 2023 Feb 16;61(2):2200840. doi: 10.1183/13993003.00840-2022. Print 2023 Feb. Eur Respir J. 2023. PMID: 36229053
-
Gut microbiota regulates circadian oscillation in hepatic ischemia-reperfusion injury-induced cognitive impairment by interfering with hippocampal lipid metabolism in mice.Hepatol Int. 2023 Dec;17(6):1645-1658. doi: 10.1007/s12072-023-10509-w. Epub 2023 Apr 1. Hepatol Int. 2023. PMID: 37004699 Free PMC article.
-
Curcumin-driven reprogramming of the gut microbiota and metabolome ameliorates motor deficits and neuroinflammation in a mouse model of Parkinson's disease.Front Cell Infect Microbiol. 2022 Aug 10;12:887407. doi: 10.3389/fcimb.2022.887407. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36034698 Free PMC article.
-
Gut-Liver Axis: Modulating the Gut Microbiota and Its Metabolic Products as a Potential Therapeutic Strategy for the Treatment of Hepatic Ischemia-Reperfusion Injury.Discov Med. 2024 Oct;36(189):1955-1972. doi: 10.24976/Discov.Med.202436189.181. Discov Med. 2024. PMID: 39463217 Review.
-
Assessing the impact of gut microbiota and metabolic products on acute lung injury following intestinal ischemia-reperfusion injury: harmful or helpful?Front Cell Infect Microbiol. 2024 Dec 2;14:1491639. doi: 10.3389/fcimb.2024.1491639. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39687547 Free PMC article. Review.
Cited by
-
Harnessing amino acid pathways to influence myeloid cell function in tumor immunity.Mol Med. 2025 Feb 4;31(1):44. doi: 10.1186/s10020-025-01099-4. Mol Med. 2025. PMID: 39905317 Free PMC article. Review.
-
The impact of glycolysis on ischemic stroke: from molecular mechanisms to clinical applications.Front Neurol. 2025 Jan 24;16:1514394. doi: 10.3389/fneur.2025.1514394. eCollection 2025. Front Neurol. 2025. PMID: 39926015 Free PMC article. Review.
-
Interferon-γ priming enhances the therapeutic effects of menstrual blood-derived stromal cells in a mouse liver ischemia-reperfusion model.World J Stem Cells. 2023 Sep 26;15(9):876-896. doi: 10.4252/wjsc.v15.i9.876. World J Stem Cells. 2023. PMID: 37900937 Free PMC article.
-
Study on the Role of Schisandrin B in Ameliorating Hepatic Ischemia-Reperfusion Injury by Modulating Hepatocyte Autophagy.Iran J Pharm Res. 2025 Apr 13;24(1):e157033. doi: 10.5812/ijpr-157033. eCollection 2025 Jan-Dec. Iran J Pharm Res. 2025. PMID: 40718455 Free PMC article.
-
Lactobacillus reuteri mitigates hepatic ischemia/reperfusion injury by modulating gut microbiota and metabolism through the Nrf2/HO-1 signaling.Biol Direct. 2024 Mar 18;19(1):23. doi: 10.1186/s13062-024-00462-5. Biol Direct. 2024. PMID: 38500127 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

