Neurophysiological mechanisms of error monitoring in human and non-human primates
- PMID: 36707544
- PMCID: PMC10231843
- DOI: 10.1038/s41583-022-00670-w
Neurophysiological mechanisms of error monitoring in human and non-human primates
Abstract
Performance monitoring is an important executive function that allows us to gain insight into our own behaviour. This remarkable ability relies on the frontal cortex, and its impairment is an aspect of many psychiatric diseases. In recent years, recordings from the macaque and human medial frontal cortex have offered a detailed understanding of the neurophysiological substrate that underlies performance monitoring. Here we review the discovery of single-neuron correlates of error monitoring, a key aspect of performance monitoring, in both species. These neurons are the generators of the error-related negativity, which is a non-invasive biomarker that indexes error detection. We evaluate a set of tasks that allows the synergistic elucidation of the mechanisms of cognitive control across the two species, consider differences in brain anatomy and testing conditions across species, and describe the clinical relevance of these findings for understanding psychopathology. Last, we integrate the body of experimental facts into a theoretical framework that offers a new perspective on how error signals are computed in both species and makes novel, testable predictions.
© 2023. Springer Nature Limited.
Figures
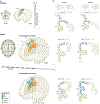



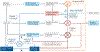
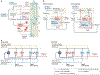
Similar articles
-
Event-related potentials associated with performance monitoring in non-human primates.Neuroimage. 2014 Aug 15;97:308-20. doi: 10.1016/j.neuroimage.2014.04.028. Epub 2014 Apr 13. Neuroimage. 2014. PMID: 24736170
-
Context-Dependent Adjustments in Executive Control of Goal-Directed Behaviour: Contribution of Frontal Brain Areas to Conflict-Induced Behavioural Adjustments in Primates.Adv Neurobiol. 2018;21:71-83. doi: 10.1007/978-3-319-94593-4_4. Adv Neurobiol. 2018. PMID: 30334220 Review.
-
Cardiac afferent activity modulates early neural signature of error detection during skilled performance.Neuroimage. 2019 Oct 1;199:704-717. doi: 10.1016/j.neuroimage.2019.04.043. Epub 2019 Apr 30. Neuroimage. 2019. PMID: 31051292
-
Trial-by-trial source-resolved EEG responses to gait task challenges predict subsequent step adaptation.Neuroimage. 2019 Oct 1;199:691-703. doi: 10.1016/j.neuroimage.2019.06.018. Epub 2019 Jun 7. Neuroimage. 2019. PMID: 31181332 Free PMC article.
-
Visual mismatch negativity (vMMN): A review and meta-analysis of studies in psychiatric and neurological disorders.Cortex. 2016 Jul;80:76-112. doi: 10.1016/j.cortex.2016.03.017. Epub 2016 Mar 31. Cortex. 2016. PMID: 27174389 Review.
Cited by
-
The Reliability of the Timed Up and Go Test among Portuguese Elderly.Healthcare (Basel). 2023 Mar 23;11(7):928. doi: 10.3390/healthcare11070928. Healthcare (Basel). 2023. PMID: 37046858 Free PMC article.
-
Neural Correlates of Cognitive Disengagement Syndrome Symptoms in Children: A Magnetoencephalography Study.Brain Sci. 2025 Jun 10;15(6):624. doi: 10.3390/brainsci15060624. Brain Sci. 2025. PMID: 40563795 Free PMC article.
-
A distributed theta network of error generation and processing in aging.Cogn Neurodyn. 2024 Apr;18(2):447-459. doi: 10.1007/s11571-023-10018-4. Epub 2023 Nov 16. Cogn Neurodyn. 2024. PMID: 38699606 Free PMC article.
-
Cognitive Control.Annu Rev Psychol. 2025 Jan;76(1):167-195. doi: 10.1146/annurev-psych-022024-103901. Epub 2024 Dec 3. Annu Rev Psychol. 2025. PMID: 39378283 Free PMC article. Review.
-
Neural mechanisms for executive control of speed-accuracy trade-off.Cell Rep. 2023 Nov 28;42(11):113422. doi: 10.1016/j.celrep.2023.113422. Epub 2023 Nov 10. Cell Rep. 2023. PMID: 37950871 Free PMC article.
References
-
-
Rabbitt PM Errors and error correction in choice-response tasks. J. Exp. Psychol 71, 264–272 (1966).
The original description of slowing after errors, revealing the existence of and a means to investigate performance monitoring.
-
-
- Logan GD & Crump MJ Cognitive illusions of authorship reveal hierarchical error detection in skilled typists. Science 330, 683–686 (2010). - PubMed
-
- Wessel JR An adaptive orienting theory of error processing. Psychophysiology 55, e13041 (2018). - PubMed
-
- Ullsperger M, Danielmeier C & Jocham G Neurophysiology of performance monitoring and adaptive behavior. Physiol. Rev 94, 35–79 (2014). - PubMed
-
- Ullsperger M Performance monitoring in neurological and psychiatric patients. Int. J. Psychophysiol 59, 59–69 (2006). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

