Self-recycling and partially conservative replication of mycobacterial methylmannose polysaccharides
- PMID: 36707645
- PMCID: PMC9883506
- DOI: 10.1038/s42003-023-04448-3
Self-recycling and partially conservative replication of mycobacterial methylmannose polysaccharides
Abstract
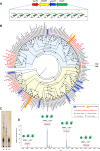
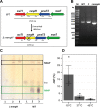
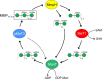
The steep increase in nontuberculous mycobacteria (NTM) infections makes understanding their unique physiology an urgent health priority. NTM synthesize two polysaccharides proposed to modulate fatty acid metabolism: the ubiquitous 6-O-methylglucose lipopolysaccharide, and the 3-O-methylmannose polysaccharide (MMP) so far detected in rapidly growing mycobacteria. The recent identification of a unique MMP methyltransferase implicated the adjacent genes in MMP biosynthesis. We report a wide distribution of this gene cluster in NTM, including slowly growing mycobacteria such as Mycobacterium avium, which we reveal to produce MMP. Using a combination of MMP purification and chemoenzymatic syntheses of intermediates, we identified the biosynthetic mechanism of MMP, relying on two enzymes that we characterized biochemically and structurally: a previously undescribed α-endomannosidase that hydrolyses MMP into defined-sized mannoligosaccharides that prime the elongation of new daughter MMP chains by a rare α-(1→4)-mannosyltransferase. Therefore, MMP biogenesis occurs through a partially conservative replication mechanism, whose disruption affected mycobacterial growth rate at low temperature.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Falkinham JO., III Current epidemiologic trends of the nontuberculous mycobacteria (NTM) Curr. Environ. Health Rep. 2016;3:161–167. - PubMed
-
- Nunes-Costa D, Alarico S, Dalcolmo MP, Correia-Neves M, Empadinhas N. The looming tide of nontuberculous mycobacterial infections in Portugal and Brazil. Tuberculosis. 2016;96:107–119. - PubMed
-
- Holt MR, Kasperbauer S. Management of extrapulmonary nontuberculous mycobacterial infections. Semin. Respir. Crit. Care Med. 2018;39:399–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

