Monocarboxylate transporter 4 involves in energy metabolism and drug sensitivity in hypoxia
- PMID: 36707650
- PMCID: PMC9883486
- DOI: 10.1038/s41598-023-28558-4
Monocarboxylate transporter 4 involves in energy metabolism and drug sensitivity in hypoxia
Abstract
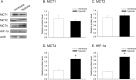
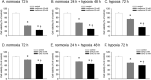
Metabolic reprogramming of cancer cells is a potential target for cancer therapy. It is also known that a hypoxic environment, one of the tumor microenvironments, can alter the energy metabolism from oxidative phosphorylation to glycolysis. However, the relationship between hypoxia and drug sensitivity, which targets energy metabolism, is not well known. In this study, A549 cells, a cell line derived from lung adenocarcinoma, were evaluated under normoxia and hypoxia for the sensitivity of reagents targeting oxidative phosphorylation (metformin) and glycolysis (α-cyano-4-hydroxycinnamic acid [CHC]). The results showed that a hypoxic environment increased the expression levels of monocarboxylate transporter (MCT) 4 and hypoxia-induced factor-1α (HIF-1α), whereas MCT1 and MCT2 expression did not vary between normoxia and hypoxia. Furthermore, the evaluation of the ATP production ratio indicated that glycolysis was enhanced under hypoxic conditions. It was then found that the sensitivity to metformin decreased while that to CHC increased under hypoxia. To elucidate this mechanism, MCT4 and HIF-1α were knocked down and the expression level of MCT4 was significantly decreased under both conditions. In contrast, the expression of HIF-1α was decreased by HIF-1α knockdown and increased by MCT4 knockdown. In addition, changes in metformin and CHC sensitivity under hypoxia were eliminated by the knockdown of MCT4 and HIF-1α, suggesting that MCT4 is involved in the phenomenon described above. In conclusion, it was shown that the sensitivity of reagents targeting energy metabolism is dependent on their microenvironment. As MCT4 is involved in some of these mechanisms, we hypothesized that MCT4 could be an important target molecule for cancer therapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Pharmacological Inhibition of MCT4 Reduces 4-Hydroxytamoxifen Sensitivity by Increasing HIF-1α Protein Expression in ER-Positive MCF-7 Breast Cancer Cells.Biol Pharm Bull. 2021;44(9):1247-1253. doi: 10.1248/bpb.b21-00030. Biol Pharm Bull. 2021. PMID: 34471053
-
Clinically relevant HIF-1α-dependent metabolic reprogramming in oropharyngeal squamous cell carcinomas includes coordinated activation of CAIX and the miR-210/ISCU signaling axis, but not MCT1 and MCT4 upregulation.Oncotarget. 2017 Feb 21;8(8):13730-13746. doi: 10.18632/oncotarget.14629. Oncotarget. 2017. PMID: 28099149 Free PMC article.
-
Oxygen tension controls the expression of the monocarboxylate transporter MCT4 in cultured mouse cortical astrocytes via a hypoxia-inducible factor-1α-mediated transcriptional regulation.Glia. 2014 Mar;62(3):477-90. doi: 10.1002/glia.22618. Epub 2013 Dec 21. Glia. 2014. PMID: 24375723
-
How does hypoxia inducible factor-1α participate in enhancing the glycolysis activity in cervical cancer?Ann Diagn Pathol. 2013 Jun;17(3):305-11. doi: 10.1016/j.anndiagpath.2012.12.002. Epub 2013 Feb 1. Ann Diagn Pathol. 2013. PMID: 23375385 Review.
-
Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function.Semin Oncol. 2014 Apr;41(2):195-216. doi: 10.1053/j.seminoncol.2014.03.002. Epub 2014 Mar 5. Semin Oncol. 2014. PMID: 24787293 Review.
Cited by
-
The emerging role of glycolysis and immune evasion in ovarian cancer.Cancer Cell Int. 2025 Mar 5;25(1):78. doi: 10.1186/s12935-025-03698-x. Cancer Cell Int. 2025. PMID: 40045411 Free PMC article. Review.
-
Sulfated and Glucuronidated Conjugates of 3-(4-Hydroxy-3-methoxyphenyl) Propionic Acid Can Promote NO Production by Elevated Ca2+ Release from the Endoplasmic Reticulum in HUVECs.ACS Omega. 2025 Jan 16;10(3):2887-2896. doi: 10.1021/acsomega.4c09008. eCollection 2025 Jan 28. ACS Omega. 2025. PMID: 39895728 Free PMC article.
-
Regulatory role and therapeutic prospect of lactate modification in cancer.Front Pharmacol. 2025 Feb 17;16:1508552. doi: 10.3389/fphar.2025.1508552. eCollection 2025. Front Pharmacol. 2025. PMID: 40034817 Free PMC article. Review.
-
Altered metabolism in cancer: insights into energy pathways and therapeutic targets.Mol Cancer. 2024 Sep 18;23(1):203. doi: 10.1186/s12943-024-02119-3. Mol Cancer. 2024. PMID: 39294640 Free PMC article. Review.
-
Lactate and lactylation in cancer.Signal Transduct Target Ther. 2025 Feb 12;10(1):38. doi: 10.1038/s41392-024-02082-x. Signal Transduct Target Ther. 2025. PMID: 39934144 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

