The role of platelets in immune-mediated inflammatory diseases
- PMID: 36707719
- PMCID: PMC9882748
- DOI: 10.1038/s41577-023-00834-4
The role of platelets in immune-mediated inflammatory diseases
Erratum in
-
Author Correction: The role of platelets in immune-mediated inflammatory diseases.Nat Rev Immunol. 2023 Jun;23(6):409. doi: 10.1038/s41577-023-00869-7. Nat Rev Immunol. 2023. PMID: 36944756 Free PMC article. No abstract available.
Abstract
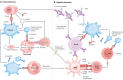
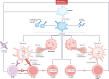
Immune-mediated inflammatory diseases (IMIDs) are characterized by excessive and uncontrolled inflammation and thrombosis, both of which are responsible for organ damage, morbidity and death. Platelets have long been known for their role in primary haemostasis, but they are now also considered to be components of the immune system and to have a central role in the pathogenesis of IMIDs. In patients with IMIDs, platelets are activated by disease-specific factors, and their activation often reflects disease activity. Here we summarize the evidence showing that activated platelets have an active role in the pathogenesis and the progression of IMIDs. Activated platelets produce soluble factors and directly interact with immune cells, thereby promoting an inflammatory phenotype. Furthermore, platelets participate in tissue injury and promote abnormal tissue healing, leading to fibrosis. Targeting platelet activation and targeting the interaction of platelets with the immune system are novel and promising therapeutic strategies in IMIDs.
© 2023. Springer Nature Limited.
Conflict of interest statement
C.R. has received consulting or speaker fees from AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb, Biogen, Eli Lilly, GlaxoSmithKline, Janssen Novartis and Pfizer and grants from Biogen, Eli Lilly and Nordic Pharma, all unrelated to this work. M.S. has received consulting fees from Sandoz, Amgen and Nordic Pharma, all unrelated to this work. The other authors report no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

