Towards full clinical trial registration and results publication: longitudinal meta-research study in Northwestern and Central Switzerland
- PMID: 36707766
- PMCID: PMC9880919
- DOI: 10.1186/s12874-023-01840-9
Towards full clinical trial registration and results publication: longitudinal meta-research study in Northwestern and Central Switzerland
Abstract
Objective: The registration of clinical trials is required by law in Switzerland. We investigated (1) the proportion of registered and prospectively registered clinical trials, (2) the availability of results for ethically approved trial protocols, (3) factors associated with increased registration, and (4) reasons for non-registration.
Design and setting: We included all clinical trials with mandatory prospective registration, which were approved by the ethics committee of Northwestern and Central Switzerland between January 1, 2016, and December 31, 2020.
Methods: We extracted relevant trial characteristics from the Swiss Business Administration System for Ethics Committees and systematically searched the International Clinical Trials Registry Platform and primary trial registries for corresponding registry entries. We used multivariable logistic regression to examine the association between trial characteristics and registration. We qualitatively assessed reasons for non-registration of trials through an email questionnaire for trial investigators.
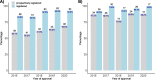
Results: Of 473 included clinical trials, 432 (91%) were registered at all and 326 (69%) were prospectively registered. While the percentages of registration and prospective registration of investigator-sponsored trials increased from 85 to 93% and from 59 to 70% over 5 years, respectively, industry-sponsored trials consistently remained at a high level of prospective registration (92 to 100%). Trials with multiple centres, higher risk category, or methodological support from the local clinical trials unit were independently associated with increased registration rates. Of 103 clinical trials completed before August 2020, results were available for 70% of industry-sponsored trials and 45% of investigator-sponsored trials as peer-reviewed journal publications or in trial registries. Most common reasons for non-registration provided by investigators were lack of time or resources (53%), lack of knowledge (22%), and lack of reminders by the ethics committee (36%).
Conclusions: In Northwestern and Central Switzerland about 10% of clinical trials remained unregistered despite the obligation by law. More support for investigators and stricter enforcement by regulators are needed to improve the transparency of investigator-sponsored trials in particular.
Keywords: Legal obligation; Non-registration reasons; Randomized controlled trials; Switzerland; Trial registration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Nonregistration, discontinuation, and nonpublication of randomized trials: A repeated metaresearch analysis.PLoS Med. 2022 Apr 27;19(4):e1003980. doi: 10.1371/journal.pmed.1003980. eCollection 2022 Apr. PLoS Med. 2022. PMID: 35476675 Free PMC article.
-
Prospective registration of trials: where we are, why, and how we could get better.J Clin Epidemiol. 2024 Dec;176:111586. doi: 10.1016/j.jclinepi.2024.111586. Epub 2024 Oct 30. J Clin Epidemiol. 2024. PMID: 39481460
-
Is Mandatory Prospective Trial Registration Working to Prevent Publication of Unregistered Trials and Selective Outcome Reporting? An Observational Study of Five Psychiatry Journals That Mandate Prospective Clinical Trial Registration.PLoS One. 2015 Aug 19;10(8):e0133718. doi: 10.1371/journal.pone.0133718. eCollection 2015. PLoS One. 2015. PMID: 26287998 Free PMC article.
-
Reliability of Trial Information Across Registries for Trials With Multiple Registrations: A Systematic Review.JAMA Netw Open. 2021 Nov 1;4(11):e2128898. doi: 10.1001/jamanetworkopen.2021.28898. JAMA Netw Open. 2021. PMID: 34724557 Free PMC article.
-
Registration and primary outcome reporting in behavioral health trials.BMC Med Res Methodol. 2022 Feb 6;22(1):41. doi: 10.1186/s12874-021-01500-w. BMC Med Res Methodol. 2022. PMID: 35125101 Free PMC article. Review.
Cited by
-
Time to publication for results of clinical trials.Cochrane Database Syst Rev. 2024 Nov 27;11(11):MR000011. doi: 10.1002/14651858.MR000011.pub3. Cochrane Database Syst Rev. 2024. PMID: 39601300
-
Protocol publication rate and comparison between article, registry and protocol in RCTs.BMC Med Res Methodol. 2025 Feb 1;25(1):31. doi: 10.1186/s12874-025-02471-y. BMC Med Res Methodol. 2025. PMID: 39893396 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical