A landscape assessment of the use of patient reported outcome measures in research, quality improvement and clinical care across a healthcare organisation
- PMID: 36707827
- PMCID: PMC9883937
- DOI: 10.1186/s12913-023-09050-1
A landscape assessment of the use of patient reported outcome measures in research, quality improvement and clinical care across a healthcare organisation
Abstract
Background: Patient reported outcome measures (PROMs) can be used by healthcare organisations to inform improvements in service delivery. However, routine collection of PROMs is difficult to achieve across an entire healthcare organisation. An understanding of the use of PROMs within an organisation can provide valuable insights on the purpose, scope and practical considerations of PROMs collection, which can inform implementation of PROMs.
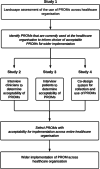
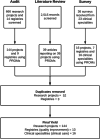
Methods: We used multiple research methods to assess the use of PROMs in research projects, data registries and clinical care across a healthcare organisation from January 2014 to April 2021. The methods included an audit of ethics applications approved by the organisation's human research ethics committee and registries which the health organisation had contributed data to; a literature review of peer-reviewed journal articles reporting on research projects conducted at the organisation; and a survey of health professionals use of PROMs in research projects, data registries and clinical care. The scope of PROMs was determined by classifying PROMs as either 'specific' to a particular disease and/or condition, or as a 'generic' measure with further classification based on the health domains they measured, using the World Health Organization International Classification Framework. Practical considerations included mode and timing of PROMs administration. Data were described using frequency and proportion.
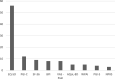
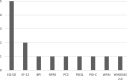
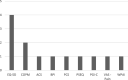
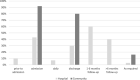
Results: PROMs were used by 22% of research projects (n = 144/666), 68% of data registries (n = 13/19), and 76% of clinical specialties in their clinical care (n = 16/21). Disease specific PROMs were most commonly used: 83% of research projects (n = 130/144), 69% of clinical registries (n = 9/13), and 75% of clinical specialties (n = 12/16). Greater than 80% of research projects, clinical registries and clinical specialties measured health domains relating to both body impairments and participation in daily life activities. The most commonly used generic PROM was the EQ-5D (research projects n = 56/144, 39%; data registries n = 5/13, 38%; clinical specialties n = 4/16, 25%). PROMs used in clinical care were mostly paper-based (n = 47/55, 85%).
Conclusions: We have elicited information on the use of PROMs to inform a health organisation wide implementation strategy. Future work will determine clinician and patient acceptability of the EQ-5D, and co-design a system for the collection of PROMs.
Keywords: Implementation science; Patient reported outcome measures; Value-based healthcare.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Using a multi-stakeholder co-design process to develop a health service organisation-wide patient reported outcome measure collection system.Qual Life Res. 2024 Mar;33(3):619-636. doi: 10.1007/s11136-023-03552-5. Epub 2023 Dec 2. Qual Life Res. 2024. PMID: 38041742
-
Collection and Reporting of Patient-reported Outcome Measures in Arthroplasty Registries: Multinational Survey and Recommendations.Clin Orthop Relat Res. 2021 Oct 1;479(10):2151-2166. doi: 10.1097/CORR.0000000000001852. Clin Orthop Relat Res. 2021. PMID: 34288899 Free PMC article.
-
Acceptability of the routine use and collection of a generic patient reported outcome measure from the perspective of healthcare staff: a qualitative study.J Patient Rep Outcomes. 2023 Jul 31;7(1):81. doi: 10.1186/s41687-023-00617-4. J Patient Rep Outcomes. 2023. PMID: 37522943 Free PMC article.
-
How to routinely collect data on patient-reported outcome and experience measures in renal registries in Europe: an expert consensus meeting.Nephrol Dial Transplant. 2015 Oct;30(10):1605-14. doi: 10.1093/ndt/gfv209. Epub 2015 May 16. Nephrol Dial Transplant. 2015. PMID: 25982327 Free PMC article.
-
Implementation of patient-reported outcome measures and patient-reported experience measures in melanoma clinical quality registries: a systematic review.BMJ Open. 2021 Feb 11;11(2):e040751. doi: 10.1136/bmjopen-2020-040751. BMJ Open. 2021. PMID: 33574144 Free PMC article.
Cited by
-
KERMIT: Performance indicators in electronic patient reported outcome measures: a modified Delphi.J Patient Rep Outcomes. 2025 Jul 2;9(1):81. doi: 10.1186/s41687-025-00898-x. J Patient Rep Outcomes. 2025. PMID: 40603694 Free PMC article.
-
Surgical outcomes and patient-centred perioperative programs.J Clin Monit Comput. 2023 Dec;37(6):1641-1643. doi: 10.1007/s10877-023-01057-7. Epub 2023 Jul 17. J Clin Monit Comput. 2023. PMID: 37460869
-
Use of patient-reported outcome measures (PROMs) in primary care-based mental health programming: an environmental scan of Alberta, Canada.BMC Prim Care. 2025 Mar 15;26(1):71. doi: 10.1186/s12875-025-02766-5. BMC Prim Care. 2025. PMID: 40089665 Free PMC article.
-
Charting a course for global progress in PIDs by 2030 - proceedings from the IPOPI global multi-stakeholders' summit (September 2023).Front Immunol. 2024 Jun 27;15:1430678. doi: 10.3389/fimmu.2024.1430678. eCollection 2024. Front Immunol. 2024. PMID: 39055704 Free PMC article.
-
A survey of practice on the use of condition-specific patient reported outcome measures with patients who have distal radius fractures.Hand Ther. 2025 Jun;30(2):63-71. doi: 10.1177/17589983241301451. Epub 2024 Dec 2. Hand Ther. 2025. PMID: 39691466 Free PMC article.
References
-
- NEJM Catalyst. What is value-based healthcare? 2017. https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0558. Accessed 12 October 2022.
-
- Porter I, Gonçalves-Bradley D, Ricci-Cabello I, Gibbons C, Gangannagaripalli J, Fitzpatrick R, et al. Framework and guidance for implementing patient-reported outcomes in clinical practice: evidence, challenges and opportunities. J Comp Eff Res. 2016;5:507–519. doi: 10.2217/cer-2015-0014. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

