Proteomic aptamer analysis reveals serum markers that characterize preclinical systemic sclerosis (SSc) patients at risk for progression toward definite SSc
- PMID: 36707842
- PMCID: PMC9881382
- DOI: 10.1186/s13075-023-02989-w
Proteomic aptamer analysis reveals serum markers that characterize preclinical systemic sclerosis (SSc) patients at risk for progression toward definite SSc
Abstract
Background: The study of molecular mechanisms characterizing disease progression may be relevant to get insights into systemic sclerosis (SSc) pathogenesis and to intercept patients at very early stage. We aimed at investigating the proteomic profile of preclinical systemic sclerosis (PreSSc) via a discovery/validation two-step approach.
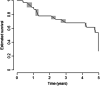
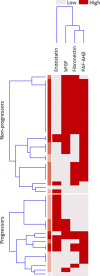
Methods: SOMAcan aptamer-based analysis was performed on a serum sample of 13 PreSSc (discovery cohort) according to 2001 LeRoy and Medsger criteria (characterized solely by Raynaud phenomenon plus a positive nailfold capillaroscopy and SSc-specific antibodies without any other sign of definite disease) and 8 healthy controls (HCs) age, gender, and ethnicity matched. Prospective data were available up to 4±0.6 years to determine the progression to definite SSc according to the EULAR/ACR 2013 classification criteria. In proteins with relative fluorescence units (RFU) > |1.5|-fold vs HCs values, univariate analysis was conducted via bootstrap aggregating models to determine the predicting accuracy (progression vs non-progression) of categorized baseline protein values. Gene Ontologies (GO terms) and Reactome terms of significant proteins at the adjusted 0.05 threshold were explored. Significant proteins from the discovery cohort were finally validated via ELISAs in an independent validation cohort of 50 PreSSc with clinical prospective data up to 5 years. Time-to-event analysis for interval-censored data was used to evaluate disease progression.
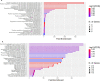
Results: In the discovery cohort, 286 out of 1306 proteins analyzed via SomaScan, were differentially expressed versus HCs. Ten proteins were significantly associated with disease progression; analysis through GO and Reactome showed differentially enriched pathways involving angiogenesis, endothelial cell chemotaxis, and endothelial cell chemotaxis to fibroblast growth factor (FGF). In the validation cohort, endostatin (HR=10.23, CI95=2.2-47.59, p=0.003) was strongly associated with disease progression, as well as bFGF (HR=0.84, CI95=0.709-0.996, p=0.045) and PAF-AHβ (HR=0.372, CI95=0.171-0.809, p=0.013) CONCLUSIONS: A distinct protein profile characterized PreSSc from HCs and proteins associated with hypoxia, vasculopathy, and fibrosis regulation are linked with the progression from preclinical to definite SSc. These proteins, in particular endostatin, can be regarded both as markers of severity and molecules with pathogenetic significance as well as therapeutic targets.
Keywords: Disease progression; Preclinical stage; Proteomic; Systemic sclerosis; Vasculopathy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Reactome pathway analysis from whole-blood transcriptome reveals unique characteristics of systemic sclerosis patients at the preclinical stage.Front Immunol. 2023 Nov 3;14:1266391. doi: 10.3389/fimmu.2023.1266391. eCollection 2023. Front Immunol. 2023. PMID: 38022564 Free PMC article.
-
Capillaroscopy changes are associated with disease progression in patients with early systemic sclerosis: A prospective study.Int J Rheum Dis. 2019 Jul;22(7):1319-1326. doi: 10.1111/1756-185X.13592. Epub 2019 May 2. Int J Rheum Dis. 2019. PMID: 31050209
-
Longitudinal global transcriptomic profiling of preclinical systemic sclerosis reveals molecular changes associated with disease progression.Rheumatology (Oxford). 2023 Apr 3;62(4):1662-1668. doi: 10.1093/rheumatology/keac492. Rheumatology (Oxford). 2023. PMID: 36040182 Free PMC article.
-
Nailfold Capillaroscopy - Its Role in Diagnosis and Differential Diagnosis of Microvascular Damage in Systemic Sclerosis.Curr Rheumatol Rev. 2013;9(4):254-60. doi: 10.2174/157339710904140417125241. Curr Rheumatol Rev. 2013. PMID: 26932290 Review.
-
Does nailfold capillaroscopy help predict future outcomes in systemic sclerosis? A systematic literature review.Semin Arthritis Rheum. 2018 Dec;48(3):482-494. doi: 10.1016/j.semarthrit.2018.02.005. Epub 2018 Feb 14. Semin Arthritis Rheum. 2018. PMID: 29602558
Cited by
-
In Silico Analysis Highlights Potential Predictive Indicators Associated with Secondary Progressive Multiple Sclerosis.Int J Mol Sci. 2024 Mar 16;25(6):3374. doi: 10.3390/ijms25063374. Int J Mol Sci. 2024. PMID: 38542346 Free PMC article.
-
Current Trends in Vascular Biomarkers for Systemic Sclerosis: A Narrative Review.Int J Mol Sci. 2023 Feb 17;24(4):4097. doi: 10.3390/ijms24044097. Int J Mol Sci. 2023. PMID: 36835506 Free PMC article. Review.
-
An international perspective on the future of systemic sclerosis research.Nat Rev Rheumatol. 2025 Mar;21(3):174-187. doi: 10.1038/s41584-024-01217-2. Epub 2025 Feb 14. Nat Rev Rheumatol. 2025. PMID: 39953141 Review.
-
Endostatin as a biomarker of systemic sclerosis: insights from a systematic review and meta-analysis.Front Immunol. 2024 Dec 23;15:1450176. doi: 10.3389/fimmu.2024.1450176. eCollection 2024. Front Immunol. 2024. PMID: 39763677 Free PMC article.
-
Immune Profiling of Patients with Systemic Sclerosis through Targeted Proteomic Analysis.Int J Mol Sci. 2023 Dec 18;24(24):17601. doi: 10.3390/ijms242417601. Int J Mol Sci. 2023. PMID: 38139427 Free PMC article.
References
-
- Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord. 2017;2:137–152.
-
- LeRoy EC, Medsger TA. Criteria for the classification of early systemic sclerosis. J Rheumatol [Internet]. 2001;28:1573–1576. - PubMed
-
- Koenig M, Joyal F, Fritzler MJ, Roussin A, Abrahamowicz M, Boire G, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud’s phenomenon to systemic sclerosis: A twenty-year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosi. Arthritis Rheum. 2008;58:3902–3912. - PubMed
-
- Bellando-Randone S, Del Galdo F, Lepri G, Minier T, Huscher D, Furst DE, et al. Progression of patients with Raynaud’s phenomenon to systemic sclerosis: a five-year analysis of the European Scleroderma Trial and Research group multicentre, longitudinal registry study for Very Early Diagnosis of Systemic Sclerosis (VEDOSS) Lancet Rheumatol. 2021;3:e834–e843. - PubMed

